推荐产品
product name
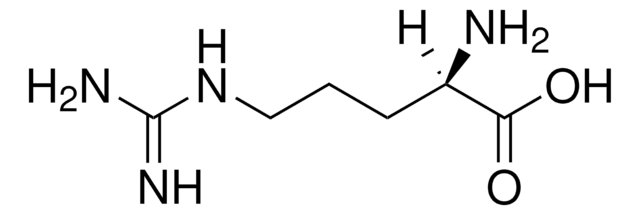
L-精氨酸, reagent grade, ≥98%
等級
reagent grade
品質等級
化驗
≥98%
形狀
powder
顏色
white
mp
222 °C (dec.) (lit.)
溶解度
H2O: 50 mg/mL
應用
cell analysis
peptide synthesis
SMILES 字串
N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s1
InChI 密鑰
ODKSFYDXXFIFQN-BYPYZUCNSA-N
基因資訊
human ... NOS1(4842) , NOS2(4843)
rat ... Ppm1a(24666)
正在寻找类似产品? 访问 产品对比指南
應用
L-精氨酸已用于研究非酶促的糖异生。它还用于研究补充L-精氨酸对心肌梗死大鼠肾脏和肝脏损伤的影响。
生化/生理作用
L-精氨酸是一种二元半必需氨基酸。它可作为肌酸酐的前体,且是大多数膳食蛋白的一种天然组成成分。
一氧化氮合成酶的底物,可转化为瓜氨酸和一氧化氮 (NO)。通过与一氧化氮相关的机理诱导胰岛素释放。
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
J.P.F. D'Mello
Amino Acids in Human Nutrition and Health (2012)
Nonenzymatic gluconeogenesis-like formation of
fructose 1,6-bisphosphate in ice
fructose 1,6-bisphosphate in ice
Christoph B. Messner
Proceedings of the National Academy of Sciences of the USA, 7403-7407 (2017)
Aerobic training and L-arginine supplement attenuates myocardial infarction-induced kidney and liver injury in rats via reduces oxidative stress
Kamal Ranjbar
Indian Heart Journal (2017)
Palaniraja Thandapani et al.
Molecular cell, 50(5), 613-623 (2013-06-12)
Motifs rich in arginines and glycines were recognized several decades ago to play functional roles and were termed glycine-arginine-rich (GAR) domains and/or RGG boxes. We review here the evolving functions of the RGG box along with several sequence variations that
Krishnan Suresh Kumar et al.
European journal of medicinal chemistry, 45(11), 5474-5479 (2010-08-21)
A new series of 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were prepared through Schiff base formation of 3-amino-2-phenyl quinazoline-4(3)H-one with various substituted carbonyl compounds. Their chemical structures were elucidated by spectral studies. Cytotoxicity and antiviral activity were evaluated against herpes simplex virus-1 (KOS), herpes simplex
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门