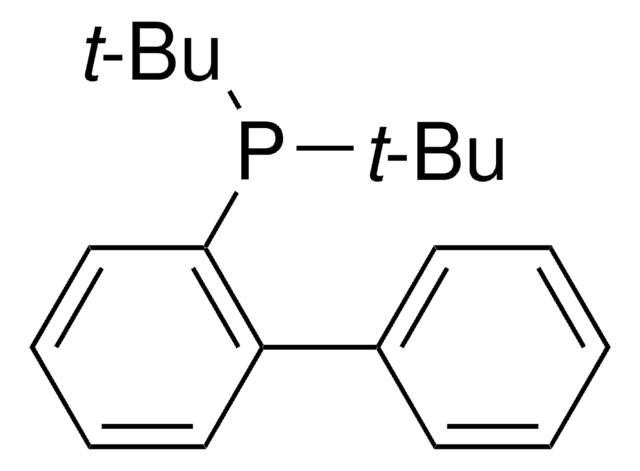
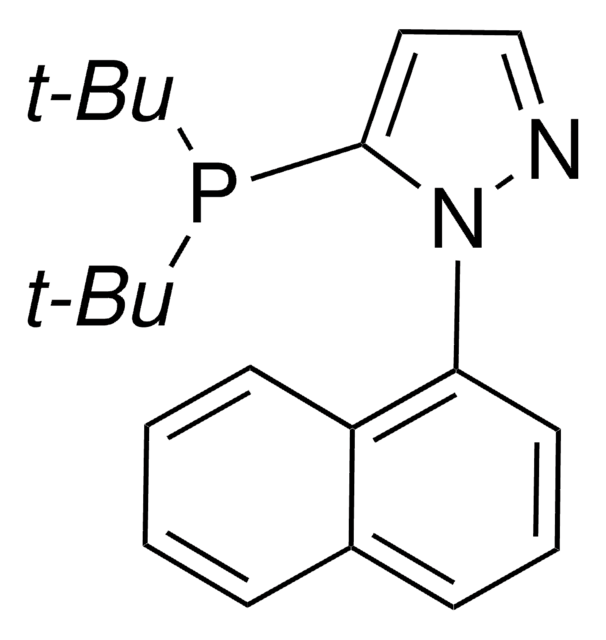
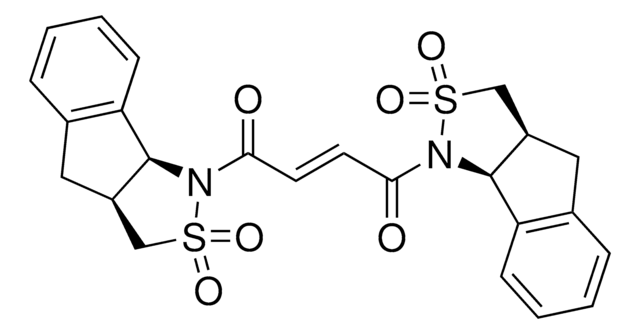
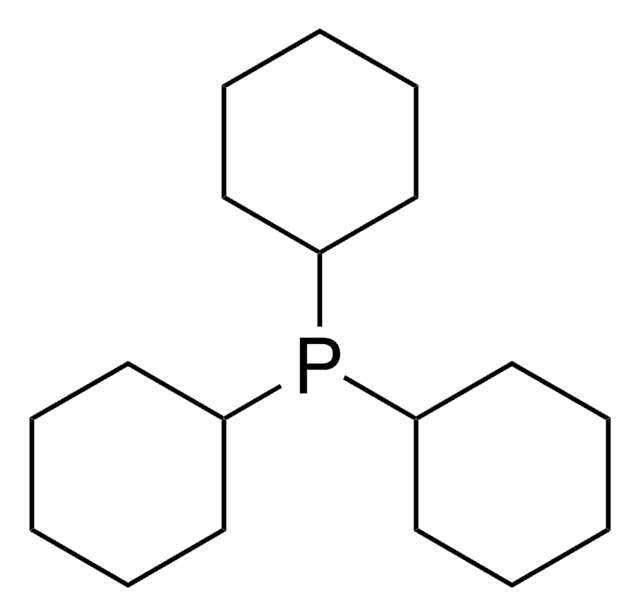
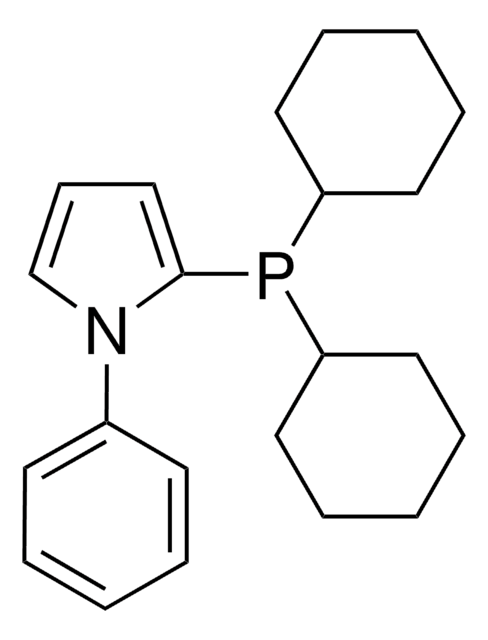
推荐产品
形狀
powder or crystals
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
reagent type: ligand
官能基
phosphine
SMILES 字串
CC(C1=CC(P(C2=CC(C(C)(C)C)=CC(C(C)(C)C)=C2)C3CCCC3)=CC(C(C)(C)C)=C1)(C)C
應用
This new aryl alkylphosphine ligand developed in Abby′s Doyle′s lab has been tailored specifically for nickel catalysis as demonstrated in the Suzuki coupling of acetals with boronic acids to generate benzylic ethers, reactions that pose challenges in Ni catalysis when using phosphine ligands developed for Pd-catalysed couplings.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Kevin Wu et al.
Nature chemistry, 9(8), 779-784 (2017-07-30)
The field of Ni-catalysed cross-coupling has seen rapid recent growth because of the low cost of Ni, its earth abundance, and its ability to promote unique cross-coupling reactions. Whereas advances in the related field of Pd-catalysed cross-coupling have been driven
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![2-{双[3,5-双(三氟甲基)苯基]膦基}-3,6-二甲氧基-2′,4′,6′-三异丙基-1,1′-联苯 95%](/deepweb/assets/sigmaaldrich/product/structures/371/999/7169f55f-ccbe-4696-bbdb-ab785b331b2e/640/7169f55f-ccbe-4696-bbdb-ab785b331b2e.png)