推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
形式
neat
儲存溫度
2-8°C
SMILES 字串
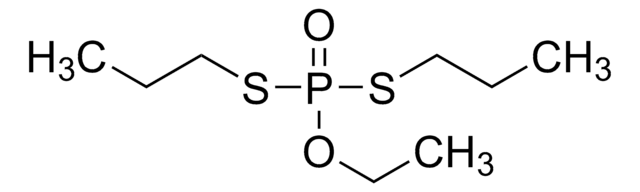
CCOP(=S)(OCC)SCCSCC
InChI
1S/C8H19O2PS3/c1-4-9-11(12,10-5-2)14-8-7-13-6-3/h4-8H2,1-3H3
InChI 密鑰
DOFZAZXDOSGAJZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
法律資訊
未找到合适的产品?
试试我们的产品选型工具.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
271.4 °F
閃點(°C)
133 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
实验方案
analytical standard; Tokuthion, technical grade, pkg of 50 mg; Crotoxyphos; Phorate; Azinphos-ethyl; Diazinon; Azinphos-methyl; Demeton-O; Dimethoate; Chlorpyrifos-methyl
Chlorobenzilate; 4-Aminobiphenyl; 2-Fluorobiphenyl; N-Nitrosopyrrolidine; 1,2,4,5-Tetrachlorobenzene; 3-Methylcholanthrene; Phenacetin
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门