推荐产品
等級
purum
品質等級
化驗
≥97.0% (GC)
形狀
liquid
折射率
n20/D 1.428 (lit.)
n20/D 1.429
bp
246-248 °C (lit.)
溶解度
ethanol: soluble 1 g/10 mL, clear, colorless
密度
0.928 g/mL at 20 °C (lit.)
官能基
anhydride
ester
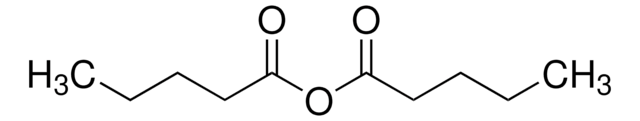
SMILES 字串
CCCCCC(=O)OC(=O)CCCCC
InChI
1S/C12H22O3/c1-3-5-7-9-11(13)15-12(14)10-8-6-4-2/h3-10H2,1-2H3
InChI 密鑰
PKHMTIRCAFTBDS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Hexanoic anhydride has been used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles and chitosan-based polymeric surfactants via N-acylation of chitosans
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Kashappa Goud Desai et al.
Drug delivery, 13(5), 375-381 (2006-08-01)
Hexanoyl chitosan was synthesized through a coupling reaction between chitosan and hexanoic anhydride. Proton nuclear magnetic resonance (1HNMR) and fourier-transform infrared (FTIR) spectroscopy studies showed the formation of hexanoyl chitosan. The nanoparticles of hexanoyl chitosan were prepared through ionotropic gelation
Rubén de Regil-Hernández et al.
Chemical & pharmaceutical bulletin, 59(9), 1089-1093 (2011-09-02)
Different green synthesis of alkyl esters of acyclovir (acyclovir prodrugs) is described. Hexanoic, decanoic, dodecanoic and tetradecanoic acyclovir esters were synthesized reacting acyclovir and the respective acid anhydride in dimethyl sulfoxide (DMSO), in solvents from renewable sources and without solvent
Moo-Yeal Lee et al.
International journal of biological macromolecules, 36(3), 152-158 (2005-07-14)
Chitosan-based polymeric surfactants (CBPSs) were prepared by N-acylation of chitosans (chitosan 10 and 500) with several acid anhydrides such as hexanoic (C6), lauric (C12), and palmitic (C16) anhydrides. Among the CBPS samples, CBPSs having a good solubility at pH 4.0
Marco Igor Valencia-Sánchez et al.
Molecular cell, 74(5), 1010-1019 (2019-04-15)
The essential histone H3 lysine 79 methyltransferase Dot1L regulates transcription and genomic stability and is deregulated in leukemia. The activity of Dot1L is stimulated by mono-ubiquitination of histone H2B on lysine 120 (H2BK120Ub); however, the detailed mechanism is not understood.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门