推荐产品
等級
technical grade
品質等級
化驗
90%
形狀
powder
bp
184-186 °C/18 mmHg (lit.)
mp
116 °C
溶解度
methanol: 50 mg/mL
應用
diagnostic assay manufacturing
hematology
histology
儲存溫度
room temp
SMILES 字串
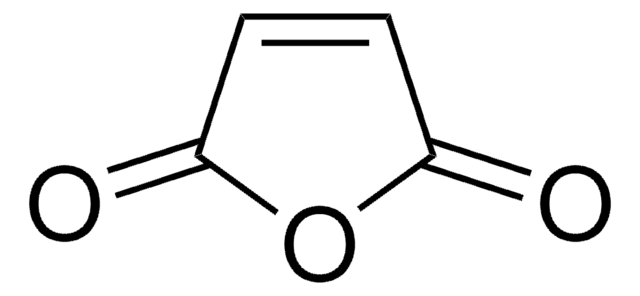
O=C1OS(C2=CC=CC=C21)(=O)=O
InChI
1S/C7H4O4S/c8-7-5-3-1-2-4-6(5)12(9,10)11-7/h1-4H
InChI 密鑰
NCYNKWQXFADUOZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
A Moulin et al.
Biochemistry, 28(15), 6340-6346 (1989-07-25)
2-Sulfobenzoic cyclic anhydride (SBA) rapidly and selectively inactivates porcine pancreatic lipase (PPL) only when added during the hydrolysis of an emulsified ester such as tributyrin or dodecyl acetate. The present data suggest that the inactivation of PPL occurs preferentially at
Christin Stegemann et al.
Rapid communications in mass spectrometry : RCM, 24(5), 599-604 (2010-02-16)
Two cyclic theta-defensin peptides were isolated from leukocytes of the hamadryas baboon, Papio hamadryas, and purified to homogeneity by gel electrophoresis and reversed-phase high-performance liquid chromatography. Both peptides had high in vitro activity against Escherichia coli, Listeria monocytogenes, methicillin-resistant Staphylococcus
Chengli Zu et al.
Rapid communications in mass spectrometry : RCM, 24(1), 120-128 (2009-12-10)
A derivatization procedure has been developed for the improved characterization of fatty alcohol ethoxylate non-ionic surfactants by liquid chromatography/mass spectrometry. The end hydroxyl group of each surfactant species was converted into an oxycarbonylbenzene-2-sulfonic acid group with 2-sulfobenzoic anhydride under mild
Modification of epsilon-amino group of lysine in proteins by acylation with pyromellitic dianhydride and o-sulphobenzoic anhydride.
A Bagree et al.
FEBS letters, 120(2), 275-277 (1980-11-03)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![N-[2-(Fmoc-氨基)-乙基]-Gly-O-tBu 盐酸盐 ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)