推荐产品
蒸汽密度
5.1 (vs air)
品質等級
蒸汽壓力
<0.01 mmHg ( 20 °C)
產品線
ReagentPlus®
化驗
99%
形狀
flakes
自燃溫度
1058 °F
expl. lim.
10.4 %
bp
284 °C (lit.)
mp
131-134 °C (lit.)
官能基
anhydride
ester
SMILES 字串
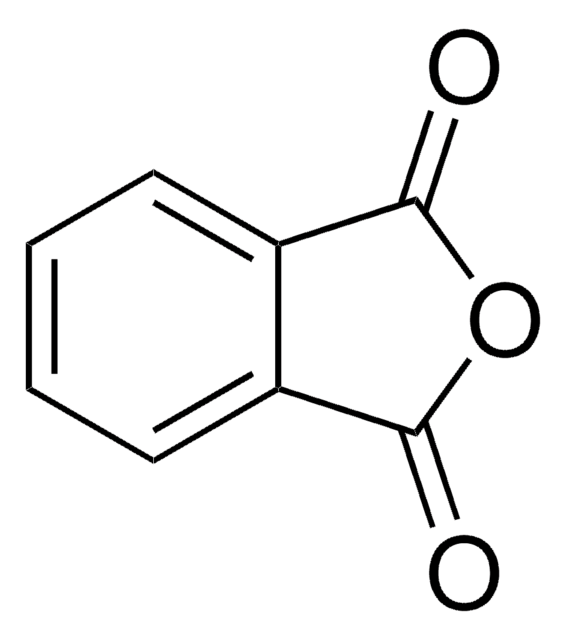
O=C1OC(=O)c2ccccc12
InChI
1S/C8H4O3/c9-7-5-3-1-2-4-6(5)8(10)11-7/h1-4H
InChI 密鑰
LGRFSURHDFAFJT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Phthalic anhydride is an industrially important chemical. It can be prepared from o-xylene or naphthalene. It is widely used for the industrial preparation of plasticizers for PVC (polyvinyl chloride).
Phthalic anhydride can be synthesized via oxidation of o-xylene, in the presence of V2O5/TiO2 monolithic catalyst supported by aluminium honeycomb.
Phthalic anhydride can be synthesized via oxidation of o-xylene, in the presence of V2O5/TiO2 monolithic catalyst supported by aluminium honeycomb.
應用
Phthalic anhydride may be used in the synthesis of the following compounds:
- pthalimide
- phenolphthalein
- anthracene
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1A - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
305.6 °F - DIN 51758
閃點(°C)
152 °C - DIN 51758
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
"Conductive monolithic catalysts: development and industrial pilot tests for the oxidation of o-xylene to phthalic anhydride"
Groppi G, et al.
Industrial & Engineering Chemistry Research, 51(22), 7590-7596 (2011)
Catalysts, kinetics and reactor design in phthalic anhydride synthesis.
Wainwright MS and Foster NR.
Catalysis Reviews: Science and Engineering, 19(2), 211-292 (1979)
Phthalic anhydride from o-xylene catalysis: science and engineering.
Nikolov V, et al.
Catalysis Reviews: Science and Engineering, 33(3-4), 319-374 (1991)
Eagleson M.
Concise Encyclopedia Chemistry, 77-77 (1994)
F Safinejad et al.
Journal of molecular modeling, 15(9), 1119-1124 (2009-02-24)
The solvent-induced changes in the optical and spectroscopic properties of 1,2-benzenedicarboxylic anhydride are studied using time dependent Hartree-Fock and density functional theory calculations within the framework of two reaction field procedures. To investigate the influence of the cavity shape, the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门