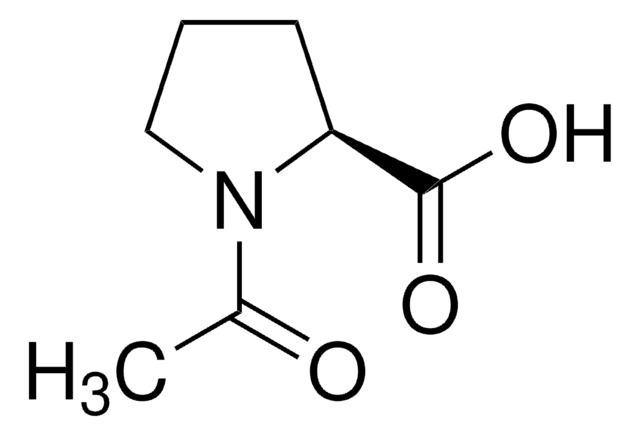
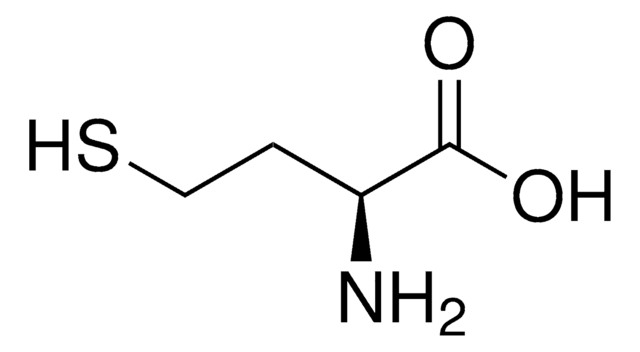
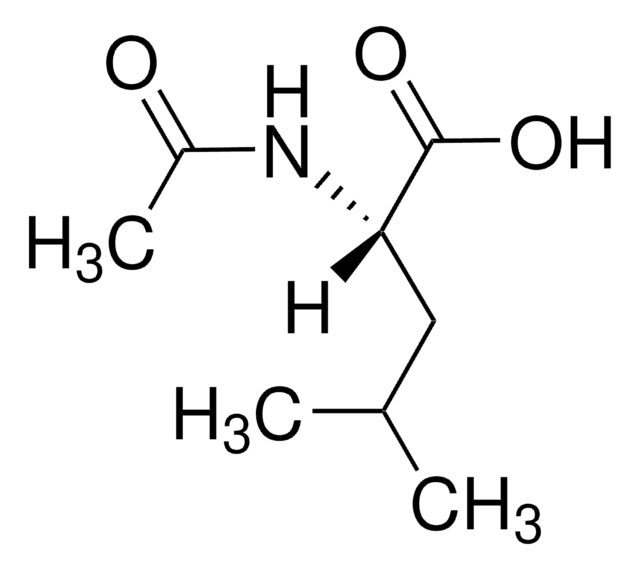
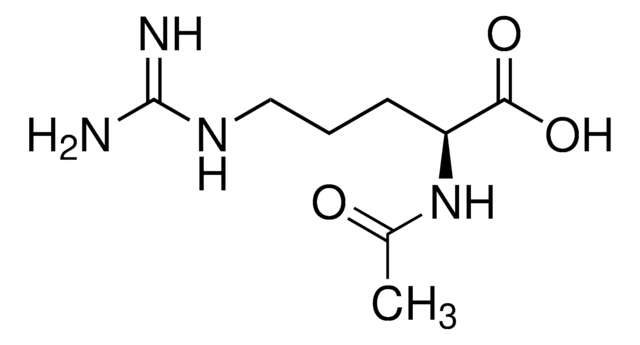
推荐产品
品質等級
化驗
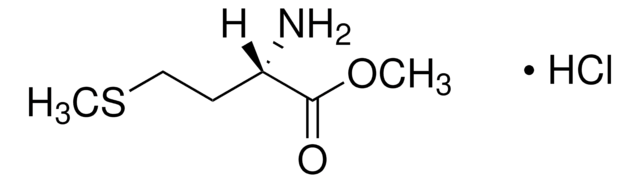
≥98.5% (T)
≥98.5% (TLC)
形狀
powder or crystals
光學活性
[α]20/D -21.0±1.0°, c = 1% in H2O
反應適用性
reaction type: solution phase peptide synthesis
mp
103-106 °C (lit.)
溶解度
methanol: 50 mg/mL, clear to very slightly hazy, colorless
應用
peptide synthesis
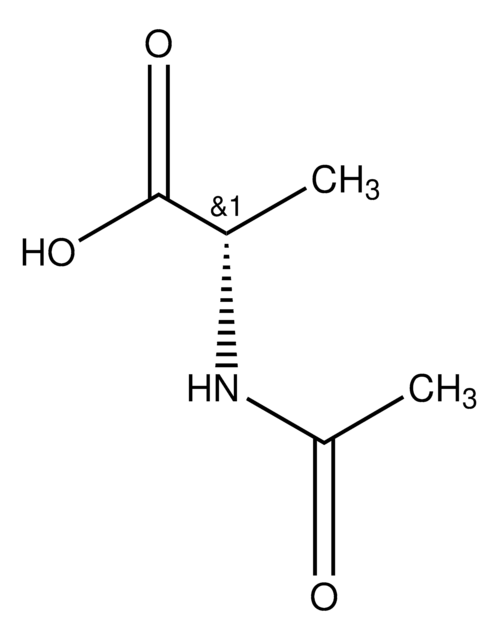
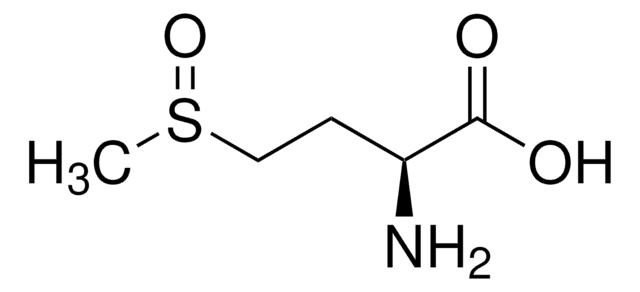
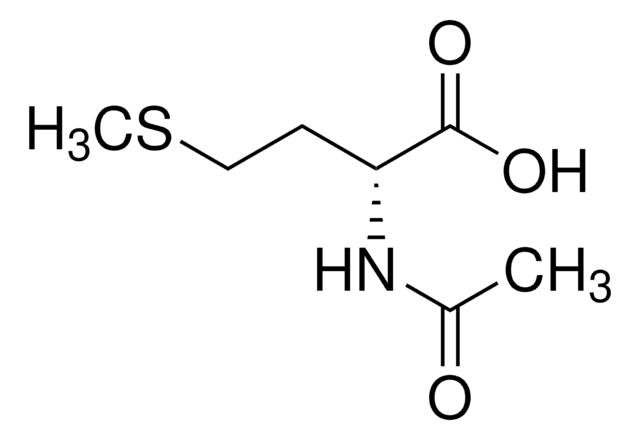
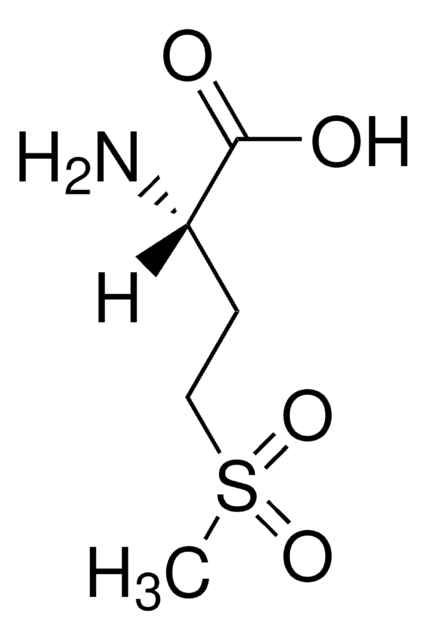
SMILES 字串
CSCC[C@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m0/s1
InChI 密鑰
XUYPXLNMDZIRQH-LURJTMIESA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Agnieszka Kołodziejczak-Radzimska et al.
Biotechnology progress, 34(3), 767-777 (2018-01-10)
Acylase I from Aspergillus melleus was immobilized on supports consisting of unmodified and modified silica. Modification was performed using 3-aminopropyltriethoxysilane (APTES) and glutaraldehyde (GA). The effectiveness of immobilization was investigated using the standard Bradford method in addition to a number
Mie R Rasmussen et al.
Journal of proteome research, 15(12), 4591-4600 (2016-11-05)
Loss-of-function mutations in the transmembrane ABCC6 transport protein cause pseudoxanthoma elasticum (PXE), an ectopic, metabolic mineralization disorder that affects the skin, eye, and vessels. ABCC6 is assumed to mediate efflux of one or several small molecule compounds from the liver
James B Thoden et al.
Biochemistry, 43(19), 5716-5727 (2004-05-12)
Divergent evolution of enzyme function is commonly explained by a gene duplication event followed by mutational changes that allow the protein encoded by the copy to acquire a new function. An alternate hypothesis is that this process is facilitated when
M J Wick et al.
Biochemical pharmacology, 37(7), 1225-1231 (1988-04-01)
Both N-hydroxy-2-acetamidofluorene (N-OH-AAF) and the heterocyclic analogue, 2-(N-hydroxyacetamido)carbazole (N-OH-AAC), were shown to be mechanism-based irreversible inhibitors (suicide inhibitors) of partially purified rat hepatic N-acetyltransferase (NAT) activity. Although N-OH-AAC exhibited an apparent first-order inactivation rate constant (ki) that was 7-fold lower
Wen-Fang Ji et al.
The journal of physical chemistry. B, 111(2), 485-489 (2007-01-12)
The amino acid oxidation mechanism has been a research focus in recent years. Although various experimental techniques have been employed to address the problem, it is still a great challenge to identify the oxidation intermediates of amino acids. To explore
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门