MABN687
Anti-β-amyloid fibril-specific, clone B10, AP Antibody
clone B10, from camel, alkaline phosphatase conjugate
别名:
Amyloid beta A4 protein, ABPP, APPI, APP, Alzheimer disease amyloid protein, Cerebral vascular amyloid peptide, CVAP, PreA4, Protease nexin-II, PN-II, N-APP2.Soluble APP-alpha, S-APP-alpha, Soluble APP-beta, S-APP-beta, C99, Beta-amyloid protein 42, Beta
登录查看公司和协议定价
所有图片(1)
About This Item
分類程式碼代碼:
12352203
eCl@ss:
32160702
NACRES:
NA.41
推荐产品
生物源
camel
品質等級
共軛
alkaline phosphatase conjugate
抗體表格
purified immunoglobulin
抗體產品種類
primary antibodies
無性繁殖
B10, monoclonal
物種活性
human
技術
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
同型
IgG
NCBI登錄號
UniProt登錄號
運輸包裝
dry ice
目標翻譯後修改
unmodified
基因資訊
human ... APP(351)
一般說明
Amyloid fibrils are naturally occurring polypeptide scaffolds with considerable importance for human health and disease. A recombinant antibody domain fragment termed B10 specifically recognizes an amyloid-specific and conformationally defined epitope. The specificity and conformational specificity have been established by various methods, including, surface plasmon resonance, immunoblots, and immunohistochemistry. All these methods demonstrate that this antibody domain fragment distinguishes Aβ amyloid fibrils from disaggregated Aβ peptide as well as from specific Aβ oligomers. The antibody domain also possesses functional activity in preventing the formation of mature amyloid fibrils by stabilizing Aβ protofibrils, and recent data suggests that the B10 antibody fragment selectively binds to Alzheimer′s Aβ(1-40) amyloid fibrils and that fibril recognition depends on positively charged residues within the B10 antigen binding site. Mutation or alternation of specific residues changes B10’s interactions with various fibril configurations, implying that the B10 conformational specificity for amyloid fibrils depends upon specific electrostatic interactions with an acidic moiety, which is common to different amyloid fibrils.
免疫原
Epitope: AB (1-40) amyloid fibrils
應用
Anti-β-amyloid fibril-specific, clone B10, AP | MABN687 is an antibody against β-amyloid fibril-specific for use in Immunohistochemistry, Dot Blot, ELISA, Immunoprecipitation, Immunofluorescence.
This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
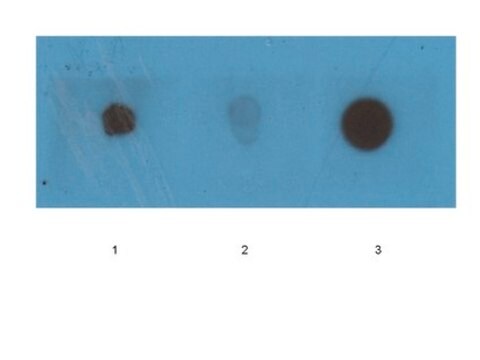
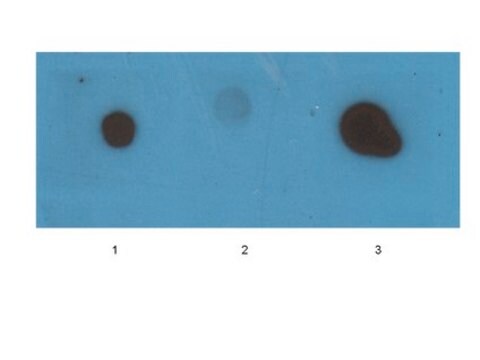
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in synthetic Aβ (1–40) peptide (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in chemically modified fibrils (Haupt, C., et al. (2011). J. Mole. Biol. 405:341-348).
Elisa Analysis: A representative lot detected β-amyloid fibril-specific in N-biotinylated Aβ (1–40) conformers (disaggregated peptide, oligomers, or fibrils) (Morgado, I., et al. (2012). PNAS. 109(31):12503-12508).
Immunohistochemistry Analysis: A representative lot detected β-amyloid fibril-specific in Hippocampal sections from Alzheimer brain tissue (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Immunoprecipitation Analysis: A representative lot detected β-amyloid fibril-specific in native soluble and dispersible fractions from the brain lysates (Upadhaya, A.R., et al. (2014). BRAIN. 1-17).
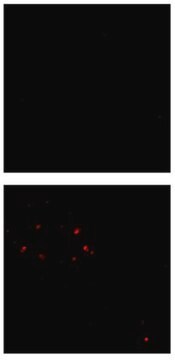
Immunofluorescence Analysis: A representative lot detected β-amyloid fibril-specific in cell culture-derived amyloid plaques (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in synthetic Aβ (1–40) peptide (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in chemically modified fibrils (Haupt, C., et al. (2011). J. Mole. Biol. 405:341-348).
Elisa Analysis: A representative lot detected β-amyloid fibril-specific in N-biotinylated Aβ (1–40) conformers (disaggregated peptide, oligomers, or fibrils) (Morgado, I., et al. (2012). PNAS. 109(31):12503-12508).
Immunohistochemistry Analysis: A representative lot detected β-amyloid fibril-specific in Hippocampal sections from Alzheimer brain tissue (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Immunoprecipitation Analysis: A representative lot detected β-amyloid fibril-specific in native soluble and dispersible fractions from the brain lysates (Upadhaya, A.R., et al. (2014). BRAIN. 1-17).
Immunofluorescence Analysis: A representative lot detected β-amyloid fibril-specific in cell culture-derived amyloid plaques (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
品質
Evaluated by Immunohistochemistry in human Alzheimer′s brain tissue.
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected β-amyloid fibril-specific in human Alzheimer′s brain tissue.
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected β-amyloid fibril-specific in human Alzheimer′s brain tissue.
外觀
Ni-NTA agarose beads and Mono Q column
Purified Camelid monoclonal IgG in buffer containing 20mM NaH2PO4, 175mM NaCl, pH8.0 without preservatives.
Note: This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
Note: This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
其他說明
Concentration: Please refer to lot specific datasheet.
未找到合适的产品?
试试我们的产品选型工具.
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
nwg
Proceedings of the National Academy of Sciences of the United States of America
Habicht, G; Haupt, C; Friedrich, RP; Hortschansky, P; Sachse, C; Meinhardt, J; Wieligmann et al.
Proceedings of the National Academy of Sciences of the USA null
Christian Haupt et al.
Journal of molecular biology, 405(2), 341-348 (2010-11-10)
Amyloid fibrils are naturally occurring polypeptide scaffolds with considerable importance for human health and disease. These supermolecular assemblies are β-sheet rich and characterized by a high structural order. Clinical diagnosis and emerging therapeutic strategies of amyloid-dependent diseases, such as Alzheimer's
Proceedings of the National Academy of Sciences of the United States of America
Morgado, I; Wieligmann, K; Bereza, M; Ronicke, R; Meinhardt, K; Annamalai, K; Baumann et al.
Proceedings of the National Academy of Sciences of the USA null
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门