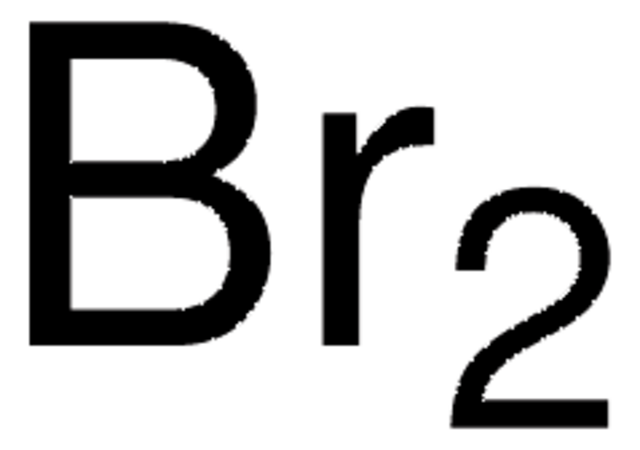推荐产品
蒸汽壓力
233 hPa ( 20 °C)
品質等級
形狀
liquid
反應適用性
reagent type: oxidant
運動黏度
0.314 cSt(20 °C)
bp
58.8 °C/1013 hPa
mp
-7.3 °C
溶解度
35 g/L
密度
3.12 g/cm3 at 20 °C (liquid)
儲存溫度
15-25°C
InChI
1S/BrH/h1H/p-1
InChI 密鑰
CPELXLSAUQHCOX-UHFFFAOYSA-M
一般說明
for synthesis
應用
Bromine can be used as an oxidizing agent for the oxidation of:
Bromine can also be used along with other co-reactants such as hexamethylphosphoric triamide (HMPA) and bis(tributyltin) oxide (HBD) to selectively oxidize secondary alcohols. However, bromine/nickel carboxylates convert 1,4-diols to γ-butyrolactones by selective oxidation of the primary alcohols.
- Primary alcohols to either aldehydes or esters and secondary alcohols to give ketones.
- Alcohols or diols to tetrahydrofurans in the presence of silver(I) salts.
- Enediol bis-trimethylsilyl ethers to α-diketones.
- Aldehydes to esters in the presence of alcohol solvents and sodium bicarbonate buffer.
- Aliphatic and aromatic thiols to disulfides.
Bromine can also be used along with other co-reactants such as hexamethylphosphoric triamide (HMPA) and bis(tributyltin) oxide (HBD) to selectively oxidize secondary alcohols. However, bromine/nickel carboxylates convert 1,4-diols to γ-butyrolactones by selective oxidation of the primary alcohols.
分析報告
Assay (iodometric)≥ 99.0 %AppearanceBrownish-red fuming liquid
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1A
儲存類別代碼
6.1B - Non-combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Preparation of disulfides by the oxidation of thiols using bromine
Wu Xiaoming, et al.
Synthetic Communications, 26(1) (1996)
Bromine as an oxidant for direct conversion of aldehydes to esters
Williams DR, et al.
Tetrahedron Letters, 29(40) (1988)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门