推荐产品
品質等級
化驗
≥95% (TLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
顏色
white
溶解度
DMSO: 25 mg/mL
運輸包裝
ambient
儲存溫度
−20°C
InChI
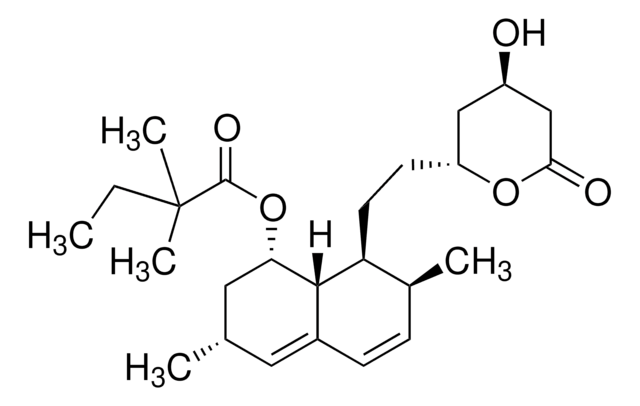
1S/C21H39NO6/c1-2-3-4-10-13-17(24)14-11-8-6-5-7-9-12-15-18(25)19(26)21(22,16-23)20(27)28/h9,12,18-19,23,25-26H,2-8,10-11,13-16,22H2,1H3,(H,27,28)/b12-9+/t18-,19+,21+/m1/s1
InChI 密鑰
ZZIKIHCNFWXKDY-GNTQXERDSA-N
一般說明
一种有效的免疫抑制剂。比环孢菌素A显示出10至100倍更强的免疫抑制活性。可有效抑制丝氨酸棕榈酰转移酶(SPT;Ki = 280 pM),从而阻断了神经酰胺的合成。破坏黑色素瘤细胞的基质粘附。在IL-2依赖性鼠细胞毒性T淋巴细胞CTLL-2中抑制细胞增殖并诱导凋亡。
生化/生理作用
产物不与ATP竞争。
可逆:否
细胞渗透性:否
靶标Ki:针对丝氨酸棕榈酰转移酶为280 pM
首要靶标
丝氨酸棕榈酰转移酶
丝氨酸棕榈酰转移酶
警告
毒性:有害(C)
重構
溶解后,等分并冷冻保存(-20°C)。储备溶液在-20°C下可稳定保存至多3个月。
其他說明
Hanada, K., et al. 2000.生物化学。Pharmacol.59, 1211.
Chen, J.K., et al. 1999.Chem. Biol. 6, 221.
Hidari, K.I.P.J., et al. 1996.J. Biol. Chem. 271, 14636.
Nakamura, S., et al. 1996.J. Biol. Chem. 271, 1255.
Mikaye, Y., et al. 1995.Biochem.Biophys.Res. Commun.211, 396.
Fujita, T., et al. 1994.J. Antibiot.47, 208.
Chen, J.K., et al. 1999.Chem. Biol. 6, 221.
Hidari, K.I.P.J., et al. 1996.J. Biol. Chem. 271, 14636.
Nakamura, S., et al. 1996.J. Biol. Chem. 271, 1255.
Mikaye, Y., et al. 1995.Biochem.Biophys.Res. Commun.211, 396.
Fujita, T., et al. 1994.J. Antibiot.47, 208.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
K Hanada et al.
Biochemical pharmacology, 59(10), 1211-1216 (2000-03-29)
In the present study, we demonstrate a model cell system for evaluating the specificity of inhibitors of serine palmitoyltransferase (SPT), the enzyme that catalyzes the first step of sphingolipid biosynthesis. The LY-B strain is a Chinese hamster ovary (CHO) cell
T Fujita et al.
The Journal of antibiotics, 47(2), 208-215 (1994-02-01)
A potent immunosuppressive activity was found in the culture broth of the fungus Isaria sinclairii (ATCC 24400). The metabolite, ISP-I ((2S,3R,4R)-(E)-2-amino-3,4-dihydroxy-2- hydroxymethyl-14-oxoeicos-6-enoic acid, myriocin = thermozymocidin) suppressed the proliferation of lymphocytes in mouse allogeneic mixed lymphocyte reaction, but had no
J K Chen et al.
Chemistry & biology, 6(4), 221-235 (1999-04-01)
Myriocin is a natural product that potently induces apoptosis of a murine cytotoxic T lymphocyte cell line (CTLL-2) and inhibits a serine palmitoyltransferase (SPT) activity that has been detected in cell extracts and is thought to initiate sphingolipid biosynthesis. Because
S Nakamura et al.
The Journal of biological chemistry, 271(3), 1255-1257 (1996-01-19)
In our previous study, the sphingosine-like immunosuppressant, ISP-1, was found to suppress the proliferation of an interleukin-2-dependent cytotoxic T cell line, CTLL-2, through the inhibition of serine palmitoyltransferase, which catalyzes the committed step of sphingolipid biosynthesis. Analysis of the effect
Y Miyake et al.
Biochemical and biophysical research communications, 211(2), 396-403 (1995-06-15)
ISP-1/myriocin is a new type of remarkably potent immunosuppressant, the structure of which is homologous to sphingosine. ISP-1/myriocin inhibited the proliferation of an IL-2-dependent mouse cytotoxic T cell line, CTLL-2, at nanomole concentrations. ISP-1/myriocin inhibits serine palmitoyltransferase activity at picomole
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门