444252
MMP-9/MMP-13 Inhibitor I
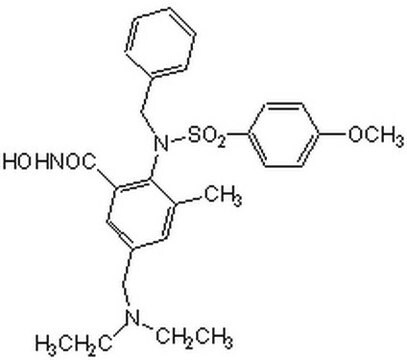
The MMP-9/MMP-13 Inhibitor I, also referenced under CAS 204140-01-2, controls the biological activity of MMP-9/MMP-13. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
别名:
MMP-9/MMP-13 Inhibitor I, N-Hydroxy-1-(4-methoxyphenyl)sulfonyl-4-(4-biphenylcarbonyl)piperazine-2-carboxamide
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥95% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
white
溶解度
methanol: 1 mg/mL
DMSO: 100 mg/mL
運輸包裝
ambient
儲存溫度
−20°C
一般說明
A cell-permeable and highly, piperazine-based potent inhibitor of MMP-9 (IC50 = 900 pM) and MMP-13 (IC50 = 900 pM). Inhibits MMP-1 and MMP-3 at much higher concentrations (IC50 = 43 nM and 23 nM, respectively). Also acts as an inhibitor of MMP-7 (IC50 = 930 nM).
A piperazine-based, cell-permeable, and highly potent inhibitor of MMP-9 (IC50 = 900 pM) and MMP-13 (IC50 = 900 pM). Inhibits MMP-1 and MMP-3 at much higher concentrations (IC50 = 43 nM and 23 nM, respectively). Also acts as an inhibitor of MMP-7 (IC50 = 930 nM).
生化/生理作用
Cell permeable: yes
Primary Target
MMP-9
MMP-9
Product does not compete with ATP.
Reversible: no
Target IC50: 900 pM against both MMP-9 and MMP-13
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Cheng, M., et al. 2000. J. Med. Chem.43, 369.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Hao Huang et al.
Cell reports, 36(2), 109363-109363 (2021-07-15)
Although activating mutations of the anaplastic lymphoma kinase (ALK) membrane receptor occur in ∼10% of neuroblastoma (NB) tumors, the role of the wild-type (WT) receptor, which is aberrantly expressed in most non-mutated cases, is unclear. Both WT and mutant proteins
Benjamin Seyer et al.
Journal of neurochemistry, 153(4), 485-494 (2019-09-27)
Ethyl2-acetylamino-7-hydroxy-4-pyridin-3-yl-4H-chromene-3-carboxylate (HFI-419), the benzopyran-based inhibitor of insulin-regulated aminopeptidase (IRAP), has previously been shown to improve spatial working and recognition memory in rodents. However, the mechanism of its cognitive-enhancing effect remains unknown. There is a close correlation between dendritic spine density
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门