437720
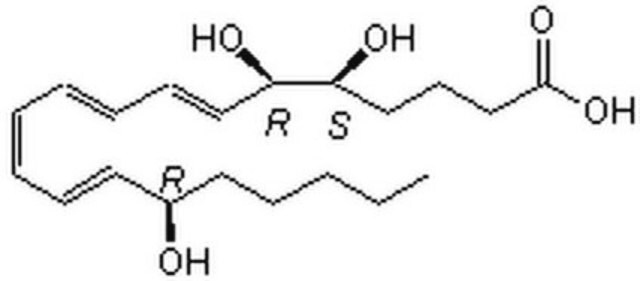
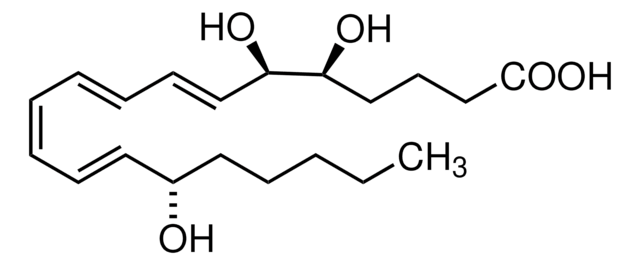
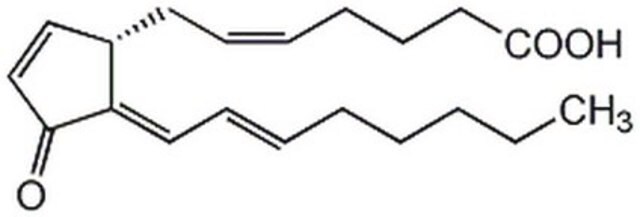
Lipoxin A4
Lipoxin A₄, CAS 89663-86-5, is a potent inhibitor of cytotoxic activity of human natural killer cells. Shown to be as potent as LTB4 in stimulating human neutrophils to generate superoxides.
别名:
Lipoxin A4, LXA₄, 5(S),6(R),15(S)-Trihydroxyeicosa-7- trans-9- trans-11- cis-13- trans-tetraenoic Acid, LXA₄, 5(S),6(R),15(S)-Trihydroxyeicosa-7-trans-9-trans-11-cis-13-trans-tetraenoic Acid
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥95% (HPLC)
形狀
liquid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
溶解度
ethanol: soluble
運輸包裝
wet ice
儲存溫度
−70°C
SMILES 字串
O[C@H]([C@H](O)\C=C\C=C\C=C/C=C/[C@@H](O)CCCCC)CCCC(=O)O
InChI
1S/C20H32O5/c1-2-3-8-12-17(21)13-9-6-4-5-7-10-14-18(22)19(23)15-11-16-20(24)25/h4-7,9-10,13-14,17-19,21-23H,2-3,8,11-12,15-16H2,1H3,(H,24,25)/b6-4-,7-5+,13-9+,14-10+/t17-,18+,19-/m0/s1
InChI 密鑰
IXAQOQZEOGMIQS-SSQFXEBMSA-N
一般說明
Caution! Product contains volatile liquid. Keep product cold at all times.
Potent inhibitor of cytotoxic activity of human natural killer cells; shown to be as potent as LTB4 in stimulating human neutrophils to generate superoxides. Involved in contractile responses and acts as a potent activator of human protein kinase C. Stimulates rapid lipid remodeling and pertussis-sensitive release of arachidonic acid in polymorphonuclear leukocytes. Stimulates MAP kinases via activation of G protein-coupled receptors.
生化/生理作用
Cell permeable: no
Primary Target
Potent inhibitor of cytotoxic activity of human natural killer cells
Potent inhibitor of cytotoxic activity of human natural killer cells
Product does not compete with ATP.
Reversible: no
警告
Toxicity: Flammable (J)
外觀
In Ethanol.
重構
Following initial thaw, aliquot and freeze (-70°C).
其他說明
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
McMahon, B., et al. 2000. J. Biol. Chem. 275, 27566.
Soyombo, O., et al. 1994. Allergy 49, 230.
Flore, S., et al. 1992. J. Biol. Chem.267, 16168.
Badr, K.F., et al. 1989. Proc. Natl. Acad. Sci. USA86, 3438.
Soyombo, O., et al. 1994. Allergy 49, 230.
Flore, S., et al. 1992. J. Biol. Chem.267, 16168.
Badr, K.F., et al. 1989. Proc. Natl. Acad. Sci. USA86, 3438.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Eye Irrit. 2 - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
O Soyombo et al.
Allergy, 49(4), 230-234 (1994-04-01)
Lipoxins are trihydroxytetraene metabolites derived through a double lipoxygenation of arachidonic acid. Lipoxin A4 (LXA4) was prepared by total chemical synthesis, and its capacity to modulate eosinophil migration has been evaluated. LXA4 is a weak and partial chemotactic agent; at
K F Badr et al.
Proceedings of the National Academy of Sciences of the United States of America, 86(9), 3438-3442 (1989-05-01)
Lipoxin A4 (LXA4) was competitive with [3H]leukotriene D4 (LTD4) for specific binding to cultured rat glomerular mesangial cells. Half-maximal inhibition was obtained with 100 nM LXA4, compared with 10 nM for unlabeled LTD4. At 10 and 50 nM LXA4 induced
B McMahon et al.
The Journal of biological chemistry, 275(36), 27566-27575 (2000-06-28)
The lipoxygenase-derived eicosanoids leukotrienes and lipoxins are well defined regulators of hemeodynamics and leukocyte recruitment in inflammatory conditions. Here, we describe a novel bioaction of lipoxin A(4) (LXA(4)), namely inhibition of leukotriene D(4) (LTD(4))-induced human renal mesangial cell proliferation, and
S Fiore et al.
The Journal of biological chemistry, 267(23), 16168-16176 (1992-08-15)
Lipoxin A4 stimulates rapid lipid remodeling and a pertussis toxin-sensitive release of arachidonic acid in polymorphonuclear leukocytes (PMN) (Nigam, S., Fiore, S., Luscinskas, F.W., and Serhan, C.N. (1990) J. Cell. Physiol. 143, 512-523) and has been shown to inhibit leukocyte
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门