324521
Eeyarestatin I
A cell-permeable oxo-imidazolidinyl-hydroxyurea that localizes to ER, where it interacts with AAA ATPase p97 via its nitrofuran-containing moiety, without exhibiting affinity toward Hsp70 / ATPase NSF
别名:
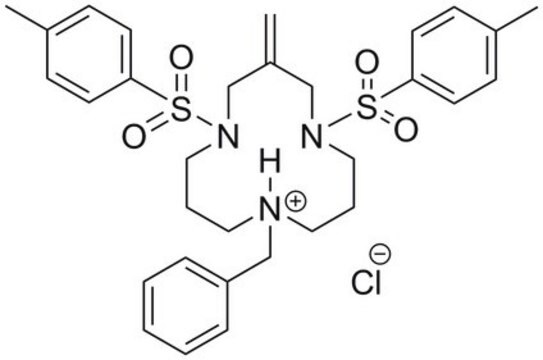
Eeyarestatin I, 1-(4-Chloro-phenyl)-3-(3-(4-chloro-phenyl)-5,5-dimethyl-1-(3-(5-nitro-furan-2-yl)-allyldiene-hydrazinocarbonylmethyl)-2-oxo-imidazolidin-4-yl)-1-hydroxyl-urea, EerI, ES1, Valosin-containing Protein Inhibitor II, VCP Inhibitor II, ERAD Inhibitor II, p97 Inhibitor II
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥90% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
light yellow-orange
溶解度
DMSO: 100 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
InChI
1S/C27H25Cl2N7O7/c1-27(2)24(35(40)25(38)31-19-9-5-17(28)6-10-19)34(20-11-7-18(29)8-12-20)26(39)33(27)16-22(37)32-30-15-3-4-21-13-14-23(43-21)36(41)42/h3-15,24,40H,16H2,1-2H3,(H,31,38)(H,32,37)
InChI 密鑰
JTUXTPWYZXWOIB-UHFFFAOYSA-N
一般說明
A cell-permeable oxo-imidazolidinyl-hydroxyurea that preferentially localizes to ER, where it interacts with AAA (ATPase associated with diverse cellular activities) ATPase p97 (Kd = 5 - 10 µM) via its nitrofuran-containing moiety, without exhibiting affinity toward Hsp70 or AAA ATPase NSF (N-methylmaleimide-sensitive factor). Evidence indicates that EerI cellular metabolite, but not EerI itself, is responsible for the inhibition of ER membrane translocon sec61 complex-mediated transfer of newly synthesized polypeptide, resulting in a blockage of ER-mediated posttranslational glycosylation and signal peptide removal (Effective conc. = 8 µM in HepG2 and HeLa cultures). Ether independent or as a consequence of the early effect on sec61 complex, EerI culture treatment also induces a selective dissociation of an 180 kDa protein from the atx3-containing p97/VCP (Valosin-containing protein) complex and a blockage of the complex-associated deubiquitination of ERAD (ER-associated protein degradation) substrates in a reversible manner, resulting in an accumulation of polyubiquitinated proteins (Effective conc. = 10 µM in A9 and 293T cultures). EerI culture treatment (10 µM) is also demonstrated to selectively induce cytotoxicity in lymphoid cell lines, BJAB, HBL-2, JEKO-1, Jurkat, KMS-12, MINO, as well as primary leukemia cells from CLL (chronic lymphocytic leukemia) patients, but not PBMC from healthy donors, by upregulating the BH3-only pro-apoptotic protein NOXA in cancer cells via ATF3/4 activation and a downregulation of H2A ubiquitination. Unlike DBeQ (Cat. No. 506190), EeRI does not inhibit p97 ATPase activity.
A cell-permeable oxo-imidazolidinyl-hydroxyurea whose cellular metabolite is shown to effectively inhibit the ER membrane translocon sec61 complex-mediated transfer of newly synthesized polypeptide, resulting in a blockage of ER-mediated posttranslational glycosylation and signal peptide removal (Effective conc. = 8 µM in HepG2 and HeLa cultures). Ether independent or as a consequence of the early effect on sec61 complex, EerI culture treatment also induces a selective dissociation of an 180 kDa protein from the atx3-containing p97/VCP (Valosin-containing protein) ATPase complex and a blockage of the p97 complex-associated deubiquitination of ERAD (ER-associated protein degradation) substrates in a reversible manner, resulting in an accumulation of polyubiquitinated proteins (Effective conc. = 10 µM in A9 and 293T cultures). EerI culture treatment (10 µM) is also demonstrated to selectively induce cytotoxicity in lymphoid cell lines, BJAB, HBL-2, JEKO-1, Jurkat, KMS-12, MINO, as well as primary leukemia cells from CLL (chronic lymphocytic leukemia) patients, but not PBMC from healthy donors, by upregulating the BH3-only pro-apoptotic protein NOXA in cancer cells via ATF3/4 activation and a downregulation of H2A ubiquitination.
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
It is recommended to prepare a stock solution for 3 month use each time and store the unreconstituted material in solid form, protected from moisture and preferably under inert gas, for best long-term stability during storage.
其他說明
Chou, T.F., et al. 2011. Proc. Natl. Acad. Sci. USA108, 4834.
Wang. Q, et al. 2010. PLoS ONE5, e15479.
Cross, B.C.S., et al. 2009. J. Cell. Sci.122, 4393.
Wang, Q., et al. 2009. Proc. Natl. Acad. Sci. USA106, 2200.
Wang, Q., et al. 2008. J. Biol. Chem.283, 7445.
Fiebiger, E., et al. 2004. Mol. Biol. Cell15, 1635.
Wang. Q, et al. 2010. PLoS ONE5, e15479.
Cross, B.C.S., et al. 2009. J. Cell. Sci.122, 4393.
Wang, Q., et al. 2009. Proc. Natl. Acad. Sci. USA106, 2200.
Wang, Q., et al. 2008. J. Biol. Chem.283, 7445.
Fiebiger, E., et al. 2004. Mol. Biol. Cell15, 1635.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门