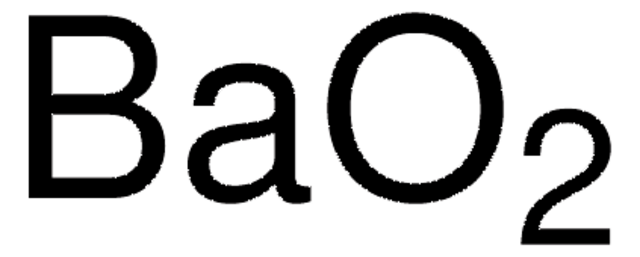推荐产品
一般說明
Barium peroxide (BaO2) is commonly used as a strong oxidizing agent in chemical industries due to its high reactivity and high storage stability under inert conditions. It is also used in fireworks because it produces a bright green color when ignited.
應用
Barium peroxide can also be used in the synthesis of hexagonal barium ferrite magnets (BaFe12O19) by SHS reaction (self-propagating high-temperature synthesis) in the presence of Fe and Fe2O3. Barium ferrite magnetic components are the most commonly used in a wide range of products, from televisions and computer monitors to kitchen cabinet magnetic clasps.
BaO2 can also be used as a:
It can also find application in various other fields such as CO2 adsorption, disinfection, luminol-driven chemiluminescence, and wastewater treatment.
BaO2 can also be used as a:
- Bleaching agent in industrial bleach applications.
- Catalyst to start an aluminothermic reaction.
- Oxidizer in various pyrotechnic mixtures.
- Green flare in tracer ammunition.
It can also find application in various other fields such as CO2 adsorption, disinfection, luminol-driven chemiluminescence, and wastewater treatment.
法律資訊
Technipur is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
儲存類別代碼
5.1A - Strongly oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Hydrogen Peroxide
Kirk-Othmer Encyclopedia of Chemical Technology (2001)
Binary and ternary pyrotechnic systems of Mn and/or Mo and BaO2 and/or SrO2: Part 2. Combustion studies
Drennan RL
Thermochimica Acta, 208, 223-246 (1992)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门