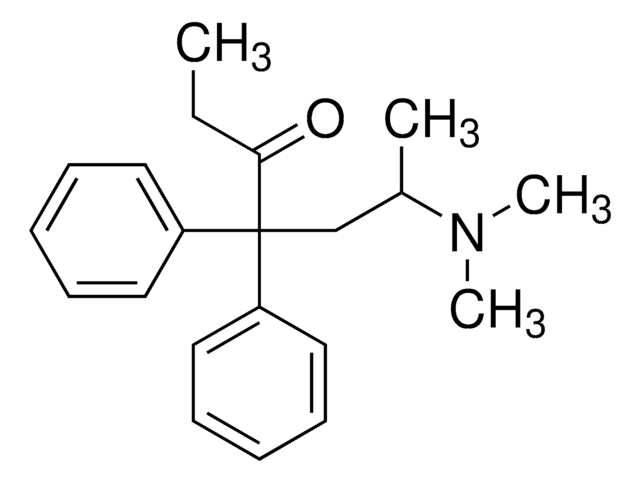
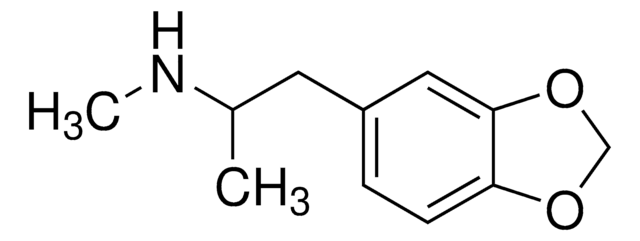
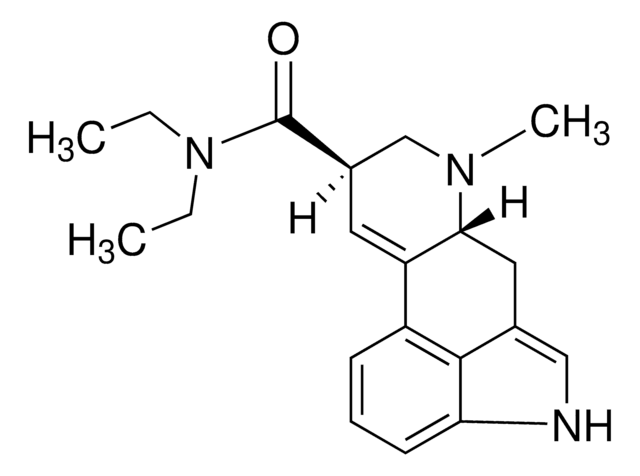
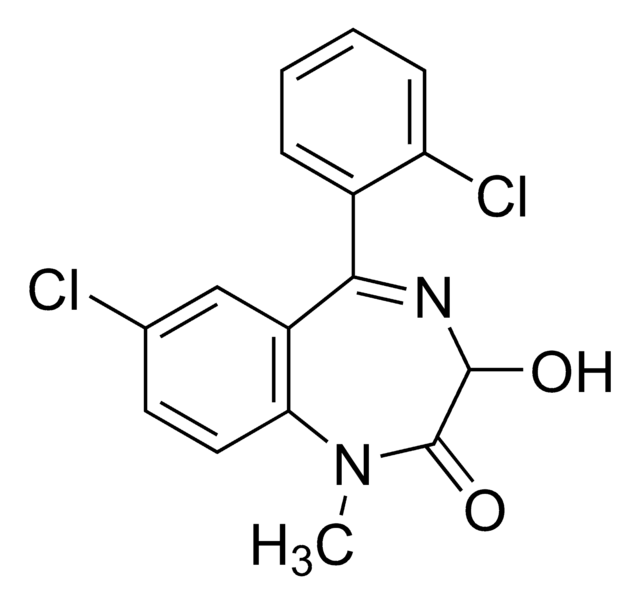
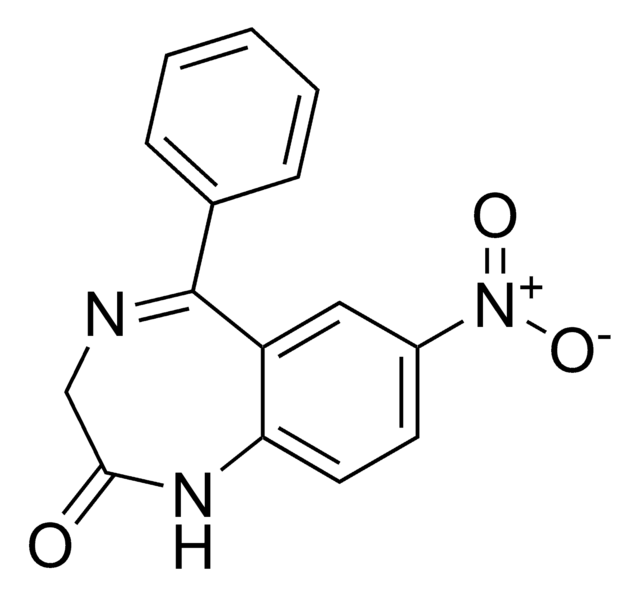
推荐产品
等級
certified reference material
品質等級
形狀
liquid
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
濃度
1 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
−20°C
SMILES 字串
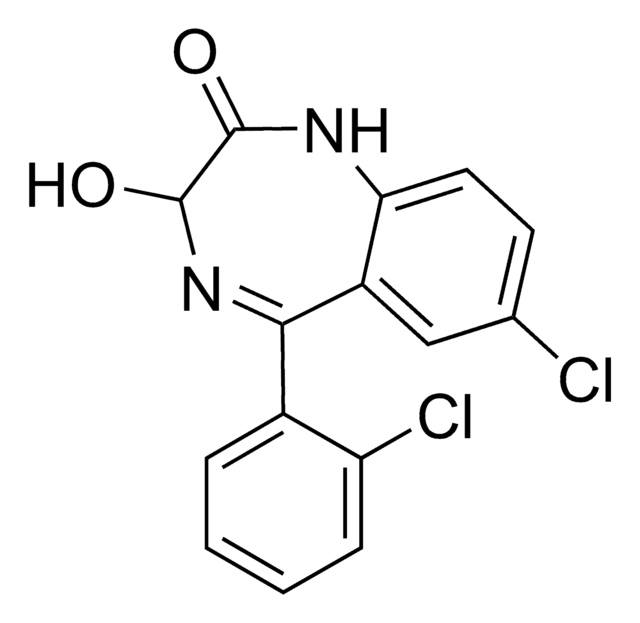
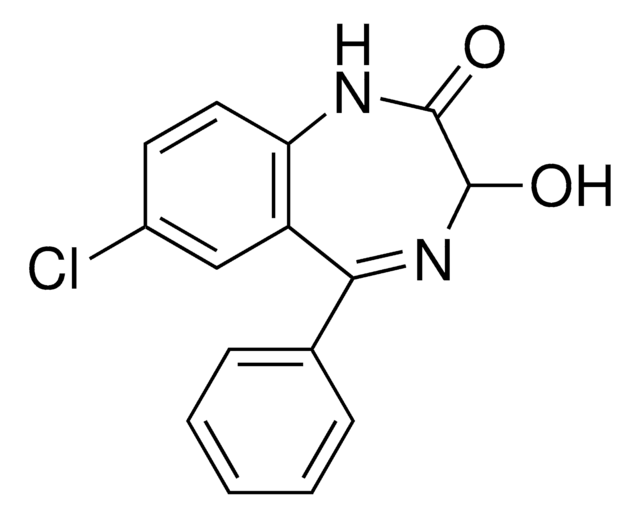
Clc1ccc2NC(=O)CN=C(c3ccccc3)c2c1
InChI
1S/C15H11ClN2O/c16-11-6-7-13-12(8-11)15(17-9-14(19)18-13)10-4-2-1-3-5-10/h1-8H,9H2,(H,18,19)
InChI 密鑰
AKPLHCDWDRPJGD-UHFFFAOYSA-N
一般說明
去甲西泮是一种苯二氮卓类药物,用于治疗焦虑症和恐慌症。该分析标准物质适用于LC/MS或GC/MS应用,以进行临床毒理学研究、法医分析或尿液药物检测。去甲西泮也是苯二氮卓类药物地西泮和氯氮卓的主要尿液代谢产物。
推薦產品
在我们的NMR在线平台ChemisTwin®上可以找到本品对应的数字化标准物质。您可使用ChemisTwin®上的数字等效品鉴定您的样品并进行定量分析(使用数字化外标)。可查看该物质的NMR谱图,只需点击几次鼠标,就能进行在线样品比对。欢迎点击了解更多,开启免费试用之旅。
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes,Central nervous system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
其他客户在看
Craig Lehmann et al.
Journal of clinical pharmacology, 48(4), 436-444 (2008-02-02)
A diazepam 10-mg autoinjector was evaluated in bioequivalence and dose proportionality studies; both involved 24 young, healthy subjects and used randomized, open-label, 2-treatment, 2-period crossover designs with a 3-week washout period between treatments. The bioequivalence study compared a single diazepam
Shanlin Fu et al.
Journal of analytical toxicology, 34(5), 243-251 (2010-06-10)
beta-Glucuronidase is an enzyme often employed to de-conjugate beta-glucuronides during urinary drug testing for benzodiazepines. It is commonly accepted that use of beta-glucuronidase is a preferred method of hydrolysis over acid-catalyzed hydrolysis, which is known to induce benzodiazepine degradation and
David A Fishbain et al.
Pain medicine (Malden, Mass.), 10(3), 565-572 (2008-11-11)
The objectives of this medicolegal case report are the following: 1) to present details of a chronic pain patient (CPP) who was placed on chronic opioid analgesic therapy (COAT), and subsequently overdosed on multiple drugs, some of which were not
Ali Acikgöz et al.
Toxicology and applied pharmacology, 234(2), 179-191 (2008-11-06)
Drug biotransformation is one of the most important parameters of preclinical screening tests for the registration of new drug candidates. Conventional existing tests rely on nonhuman models which deliver an incomplete metabolic profile of drugs due to the lack of
Joris C Verster et al.
Sleep medicine reviews, 17(2), 153-159 (2012-08-14)
The use of benzodiazepine receptor agonists can significantly impair driving performance. The aim of this review was to determine if there is a relation between blood concentrations of these drugs and the degree of driving impairment. A literature search was
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| N-905-1ML | 4061834092568 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持