推荐产品
形狀
powder
包裝
pkg of 1 × 5 mg (870706P-5mg)
製造商/商標名
Avanti Research™ - A Croda Brand 870706P
應用
lipidomics
脂質類型
coenzymes
運輸包裝
dry ice
儲存溫度
−20°C
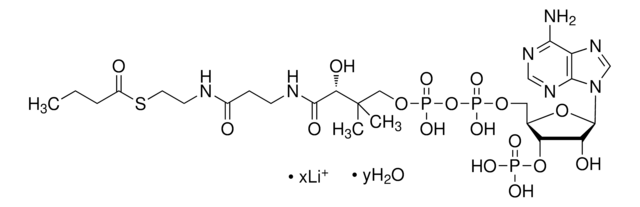
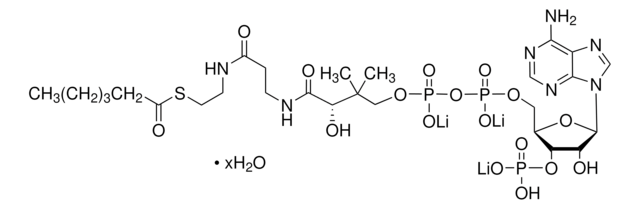
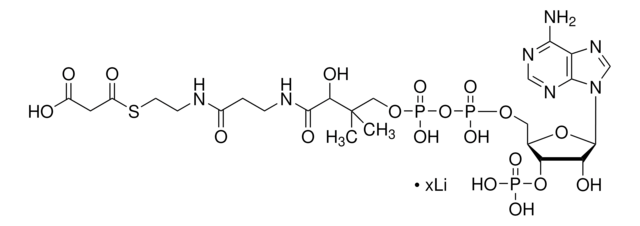
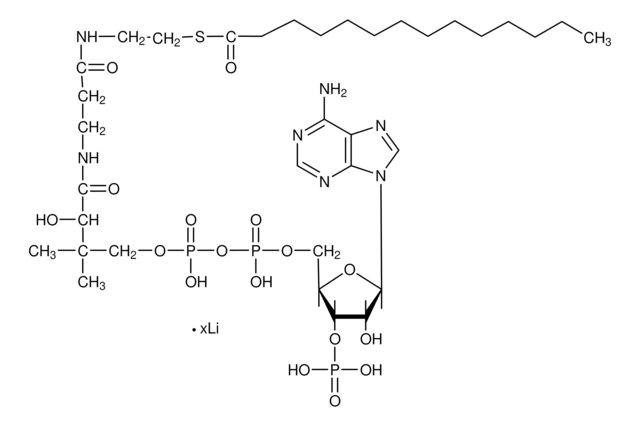
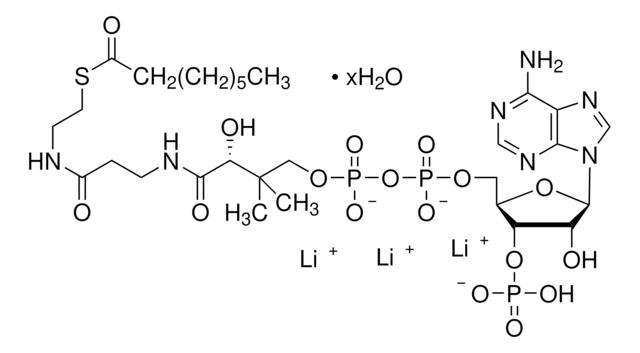
SMILES 字串
O[C@@](C(NCCC(NCCSC(CCCCC)=O)=O)=O)(C(C)(COP([O-])(OP([O-])(OC[C@H]([C@H]1OP([O-])(O)=O)O[C@H]([C@@H]1O)N2C3=C(C(N)=NC=N3)N=C2)=O)=O)C)[H].[NH4+].[NH4+].[NH4+]
InChI
1S/C27H46N7O17P3S.3H3N/c1-4-5-6-7-18(36)55-11-10-29-17(35)8-9-30-25(39)22(38)27(2,3)13-48-54(45,46)51-53(43,44)47-12-16-21(50-52(40,41)42)20(37)26(49-16)34-15-33-19-23(28)31-14-32-24(19)34;;;/h14-16,20-22,26,37-38H,4-13H2,1-3H3,(H,29,35)(H,30,39)(H,43,44)(H,45,46)(H2,28,31,32)(H2,40,41,42);3*1H3/t16-,20?,21+,22+,26-;;;/m1.../s1
InChI 密鑰
MXEFXWIYODKXEJ-WSHYHEJESA-N
應用
06:0 Coenzyme A has been used as an internal standard in ultra-high performance liquid chromatography–tandem mass spectrometry for quantitative analysis of fatty-acyl-Coenzyme A species in biological samples.
生化/生理作用
06:0 Coenzyme A, also known as hexanoyl coenzyme A, is a derivate of short-chain fatty acid hexanoate. It might be an intermediate of fatty acid metabolism. It acts as a source of N-acyl chain in the synthesis of N-hexanoyl-L-homoserine lactone (HHL) by LuxI homologue RhlI(VsmI). Hexanoyl coenzyme A also acts as a precursor for the synthesis of olivetolic acid (OLA), a polyketide metabolite. N-hexanoyl-CoA is preferred over N-octanoyl-CoA as an acyl donor substrate by GOAT (ghrelin O-acyltransferase).
包裝
5 mL Amber Glass Screw Cap Vial (870706P-5mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Y Jiang et al.
Molecular microbiology, 28(1), 193-203 (1998-05-21)
In Pseudomonas aeruginosa, synthesis of the quorum-sensing signal molecules N-butanoyl-L-homoserine lactone (BHL) and N-hexanoyl-L-homoserine lactone (HHL) requires the Luxl homologue Rhll(Vsml). By using thin-layer chromatography in conjunction with high-performance liquid chromatography (HPLC) and mass spectrometry, we show that purified Rhll
Jake M Stout et al.
The Plant journal : for cell and molecular biology, 71(3), 353-365 (2012-02-23)
The psychoactive and analgesic cannabinoids (e.g. Δ(9) -tetrahydrocannabinol (THC)) in Cannabis sativa are formed from the short-chain fatty acyl-coenzyme A (CoA) precursor hexanoyl-CoA. Cannabinoids are synthesized in glandular trichomes present mainly on female flowers. We quantified hexanoyl-CoA using LC-MS/MS and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门