推荐产品
品質等級
化驗
97%
形狀
liquid
包含
0.05% BHT as inhibitor
折射率
n20/D 1.474 (lit.)
bp
80 °C (lit.)
密度
0.841 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
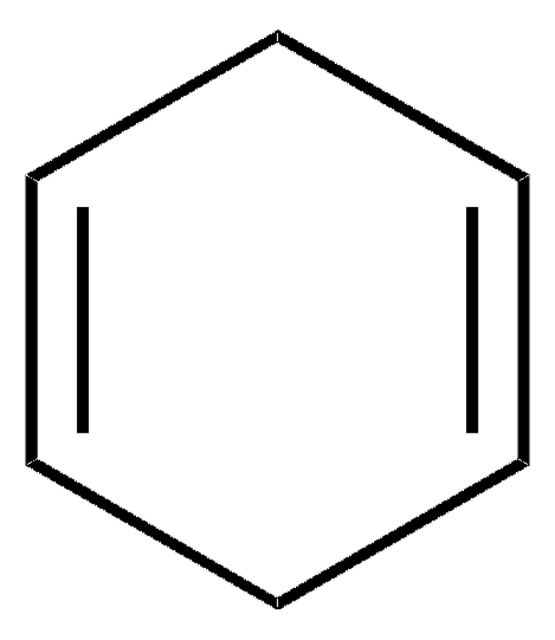
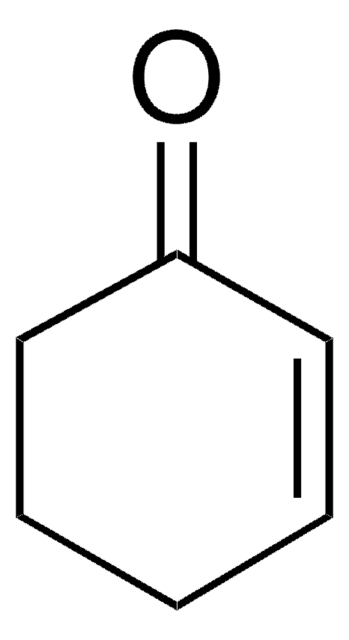
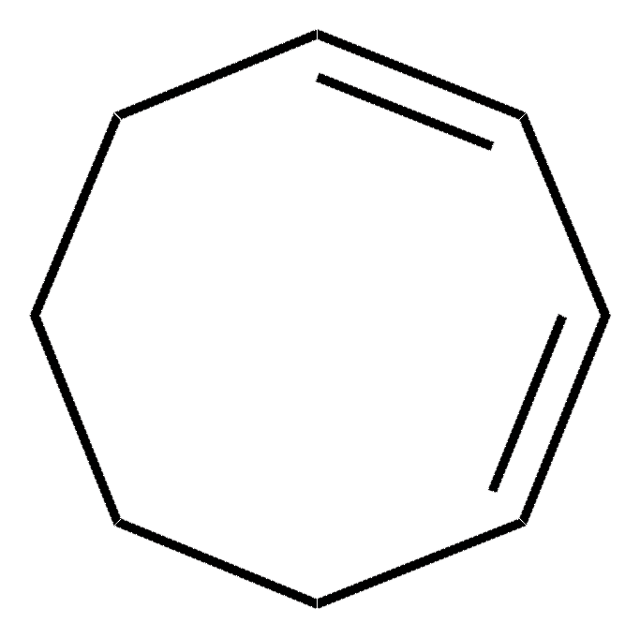
SMILES 字串
C1CC=CC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-4H,5-6H2
InChI 密鑰
MGNZXYYWBUKAII-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
1,3-环己二烯可以通过:
- 铱催化的氢自动转移与芳香醇进行C-C偶联,并通过异丙醇介导的转移氢化与醛发生偶联,从而形成羰基加成产物。
- 与n-BuLi/TMEDA系统发生活性阴离子聚合,从而形成聚环己二烯。
- 铂催化的硅烷化形成(1R,4S)-1-(二甲基苯基)-4-(4,4,5,5-四甲基-1,3,2-二氧硼戊环-2-基)-2-环己烯。
- 在作为电子转移试剂的四(氢醌)卟啉钴存在下,进行需氧钯催化的1,4-二乙酰氧基化。
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
66.0 °F
閃點(°C)
18.9 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Sarah J Ryan et al.
Journal of the American Chemical Society, 133(13), 4694-4697 (2011-03-12)
Herein we report the first all-carbon N-heterocyclic carbene-catalyzed (4 + 2) cycloaddition. The reaction proceeds with α,β-unsaturated acid fluorides and silyl dienol ethers and produces 1,3-cyclohexadienes with complete diastereocontrol (dr >20:1) while demonstrating a new type of reaction cascade exploiting
Contact formation dynamics: Mapping chemical bond formation between a molecule and a metallic probe.
Borislav Naydenov et al.
Nano letters, 6(8), 1752-1756 (2006-08-10)
We present a study that maps out chemical bond formation between a Pt-inked probe and a single 1,3-cyclohexadiene (1,3-CHD) molecule on Si(100). By separating the mechanical and electronic contributions to the current during the approach to contact, we show that
Enantioselective Platinum?Catalyzed Silicon?Boron Addition to 1, 3?Cyclohexadiene.
Gerdin M and Moberg C
Advanced Synthesis & Catalysis, 347(6), 749-753 (2005)
Marija Kotur et al.
The Journal of chemical physics, 130(13), 134311-134311 (2009-04-10)
We demonstrate the use of shaped ultrafast laser pulses in the deep ultraviolet to control the ring opening isomerization of 1,3-cyclohexadiene to form 1,3,5-hexatriene. The experiments are performed with a gas phase sample and the isomerization yield is probed with
Chemo-, regio-, and stereoselective cobalt-mediated [2+2+2] cycloaddition of alkynyl boronates to alkenes: 1,3- and 1,4-diboryl-1,3-cyclohexadienes.
Vincent Gandon et al.
Angewandte Chemie (International ed. in English), 44(43), 7114-7118 (2005-10-12)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门