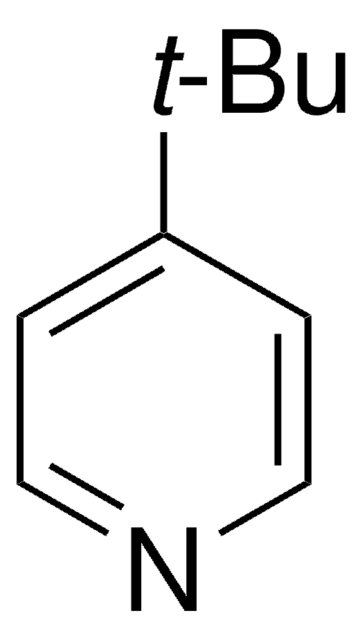
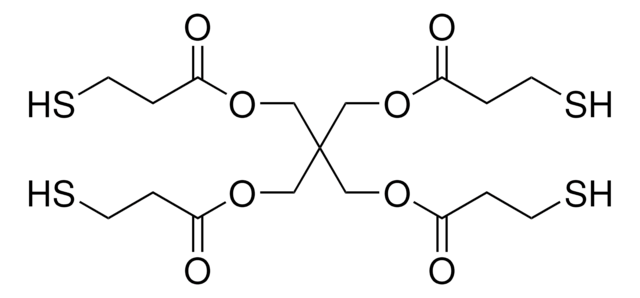
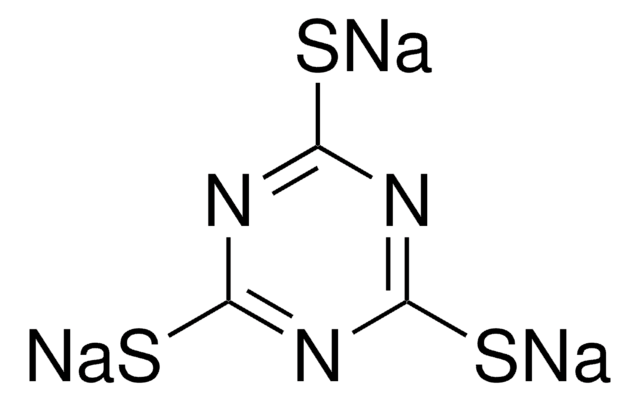
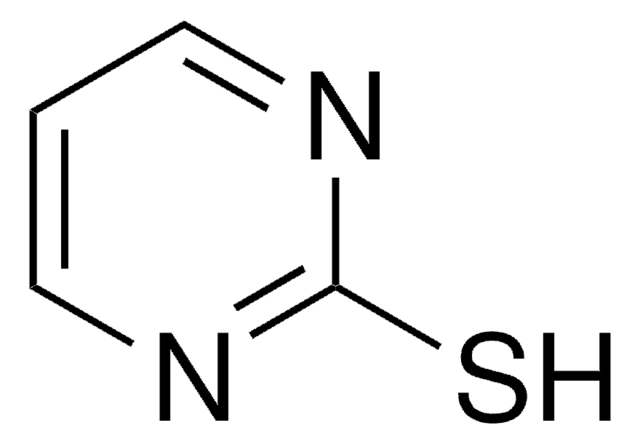
推荐产品
品質等級
化驗
≥95%
形狀
flakes
mp
141-146 °C
儲存溫度
−20°C
SMILES 字串
C1(C2=CC=CC=C2)=CN=NN=C1
InChI
1S/C9H7N3/c1-2-4-8(5-3-1)9-6-10-12-11-7-9/h1-7H
InChI 密鑰
KJZQIXWSZPPOHO-UHFFFAOYSA-N
一般說明
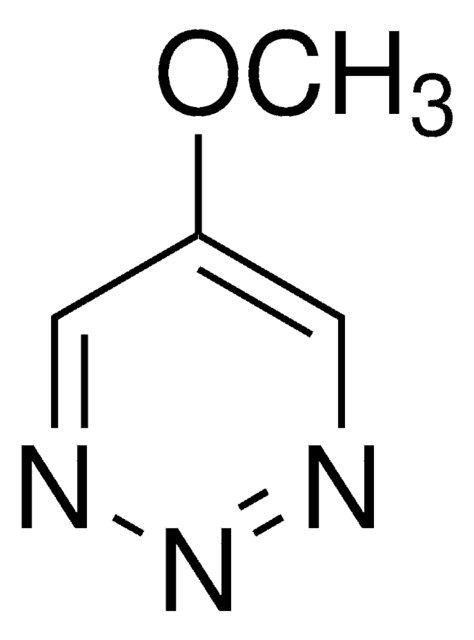
5-Phenyl-1,2,3-triazine is a phenyl triazine derivative. 5-phenyl-1,2,3-triazine exhibits electronic and nonlinear optical properties. 5-Phenyl-1,2,3-triazine can be prepared from 4-bromopyrazole. It undergoes Diels-Alder reaction with ketene acetal.
應用
The following 1,2,3-triazine was reported by Boger and coworkers to undergo an Inverse Electron Demand Diels-Alder with electron rich dienophiles to afford nitrogen-containing heterocycles, more specifically pyrimidines and novel-substituted pyridines.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Investigation of torsional barriers and nonlinear optical (NLO) properties of phenyltriazines.
Alyar H, et al.
Journal of Molecular Structure, 834, 516-520 (2007)
Erin D Anderson et al.
Journal of the American Chemical Society, 133(31), 12285-12292 (2011-07-09)
A systematic study of the inverse electron demand Diels-Alder reactions of 1,2,3-triazines is disclosed, including an examination of the impact of a C5 substituent. Such substituents were found to exhibit a remarkable impact on the cycloaddition reactivity of the 1,2,3-triazine
商品
The inverse electron demand Diels-Alder reactions of electron-deficient heterocycles are significant cycloaddition reactions for the total synthesis of natural products containing highly substituted and functionalized heteroaromatic ring systems.
The inverse electron demand Diels-Alder reactions of electron-deficient heterocycles are significant cycloaddition reactions for the total synthesis of natural products containing highly substituted and functionalized heteroaromatic ring systems.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门