推荐产品
化驗
≥90%
品質等級
形狀
powder
反應適用性
reaction type: Cross Couplings
官能基
(palladium)
phosphine
SMILES 字串
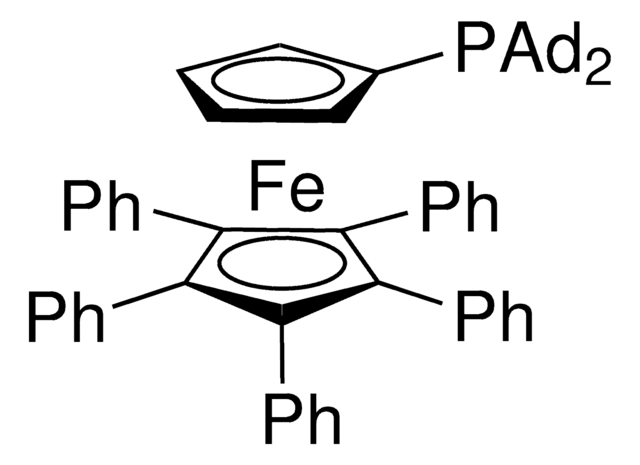
CS(O[Pd]1([P](C23C[C@H]4C[C@H](C[C@H](C4)C3)C2)(C56C[C@H]7C[C@H](C[C@H](C7)C6)C5)c8cccc8)[NH2]C(C=CC=C9)=C9C%10=C1C=CC=C%10)(=O)=O.c%11(C%12=CC=CC=C%12)c(C%13=CC=CC=C%13)c(C%14=CC=CC=C%14)c(C%15=CC=CC=C%15)c%11C%16=CC=CC=C%16.[Fe]
一般說明
The precatalysts derived from AdQPhos exhibit excellent activity towards palladium-catalyzed α-arylation reactions under milder conditions. The strength of these catalytic systems in enabling such reactions were demonstrated with the broad scope of nucleophiles, including nitroalkanes, nitriles, amides, esters, and ketones. Compared to other state-of-the-art catalysts, this system was far superior and served as the first versatile catalytic system to accommodate all these nucleophiles in this context.
Further, by merging with micellar catalysis, this catalytic system demonstrated the feasibility of enabling α-arylation reactions under mild aqueous conditions. This approach provides an environmentally benign alternative to toxic/hazardous solvents like 1,4-dioxane or NMP, bringing modern organic synthesis closer to being ideally sustainable.
Further, by merging with micellar catalysis, this catalytic system demonstrated the feasibility of enabling α-arylation reactions under mild aqueous conditions. This approach provides an environmentally benign alternative to toxic/hazardous solvents like 1,4-dioxane or NMP, bringing modern organic synthesis closer to being ideally sustainable.
應用
In addition to facilitating α-arylations, these catalysts demonstrate compatibility with various challenging cross-coupling reactions, such as Suzuki-Miyaura, Buchwald-Hartwig aminations, and C-O and C-S cross-couplings.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门