所有图片(3)
About This Item
经验公式(希尔记法):
C80H64Cl16N4O16Rh2
CAS号:
分子量:
2110.44
MDL號碼:
分類程式碼代碼:
12352101
NACRES:
NA.22
推荐产品
形狀
powder or crystals
應用
This dirhodium catalyst developed by the Davies lab can form C-C bonds at the most accessible tertiary C-H position with control of both regioselectivity and absolute configuration.
其他說明
Formation of Tertiary Alcohols from the Rhodium-Catalyzed Reactions of Donor/Acceptor Carbenes with Esters
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Enantioselective intramolecular aza-spiroannulation onto benzofurans using chiral rhodium catalysis.
Shibuta T, et al.
Heterocycles, 89, 631-639 (2014)
Liangbing Fu et al.
Organic letters, 16(11), 3036-3039 (2014-05-21)
A rhodium-catalyzed asymmetric synthesis of β-lactones via intramolecular C-H insertion into the ester group of aryldiazoacetates has been developed. The β-lactones were synthesized in high yields and with high levels of diastereo- and enantioselectivity. Halo and trifluoromethyl substituents at the
Shigeki Sato et al.
Chemical communications (Cambridge, England), (41), 6264-6266 (2009-10-15)
A versatile, highly enantiocontrolled entry to the spiro-beta-lactam core of chartellines has been developed by expanding the scope of oxidative nitrogen atom transfer methodology based on chiral Rh-nitrenoid species.
Liangbing Fu et al.
Organic letters, 20(8), 2399-2402 (2018-04-12)
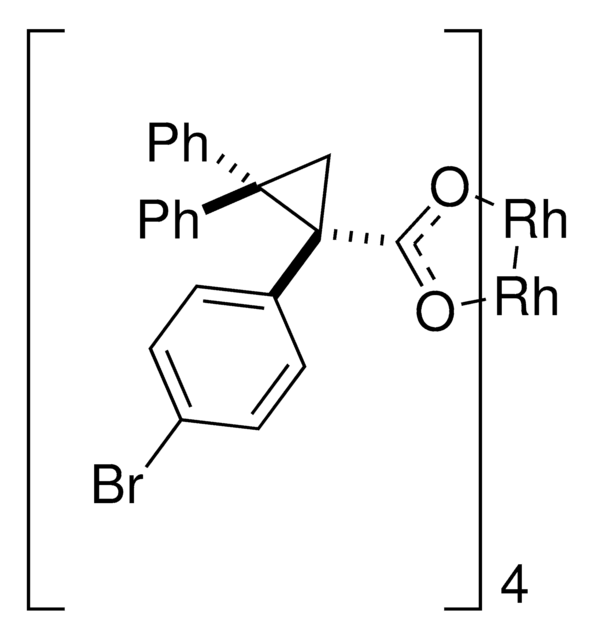
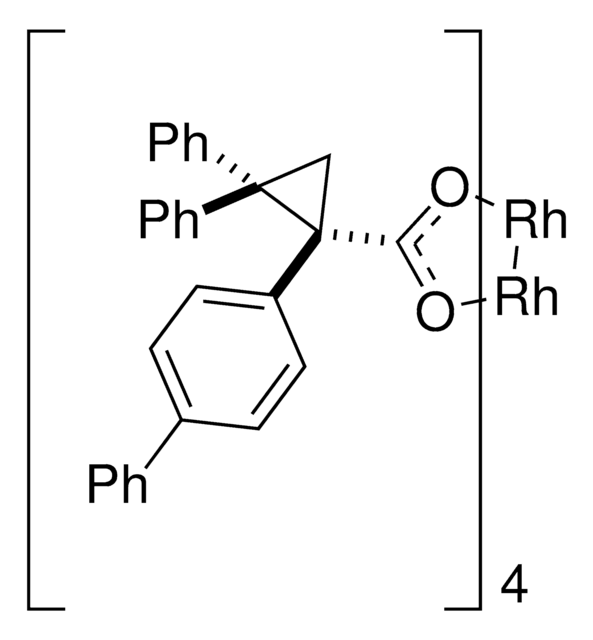
Rhodium(II)-catalyzed reactions between isopropyl acetate and trichloroethyl aryldiazoacetates result in the formation of oxirane intermediates that ring open under the reaction conditions to form tertiary alcohols. When the reaction is catalyzed by the dirhodium tetrakis(triarylcyclopropanecarboxylate) complex, Rh2( S-2-Cl,4-BrTPCP)4, the tertiary
Hengbin Wang et al.
Chemical science, 4(7), 2844-2850 (2013-09-21)
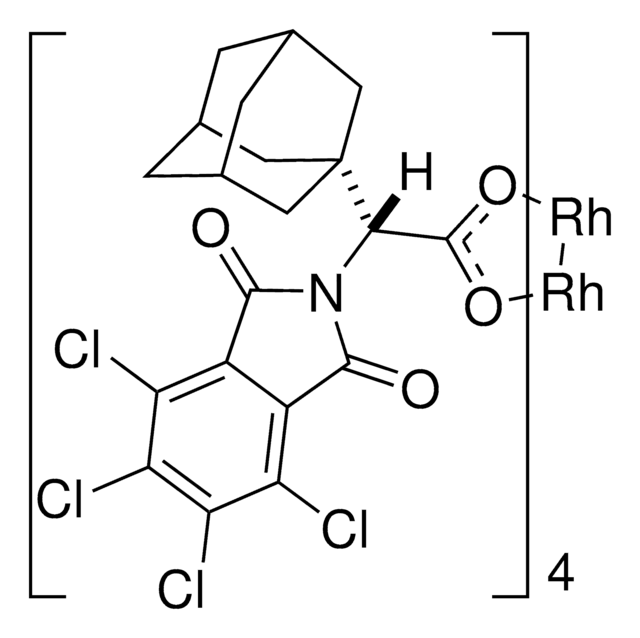
The rhodium-catalyzed reaction of electron-deficient alkenes with substituted aryldiazoacetates and vinyldiazoacetates results in highly stereoselective cyclopropanations. With adamantylglycine derived catalyst Rh2(S-TCPTAD)4, high asymmetric induction (up to 98% ee) can be obtained with a range of substrates. Computational studies suggest that
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门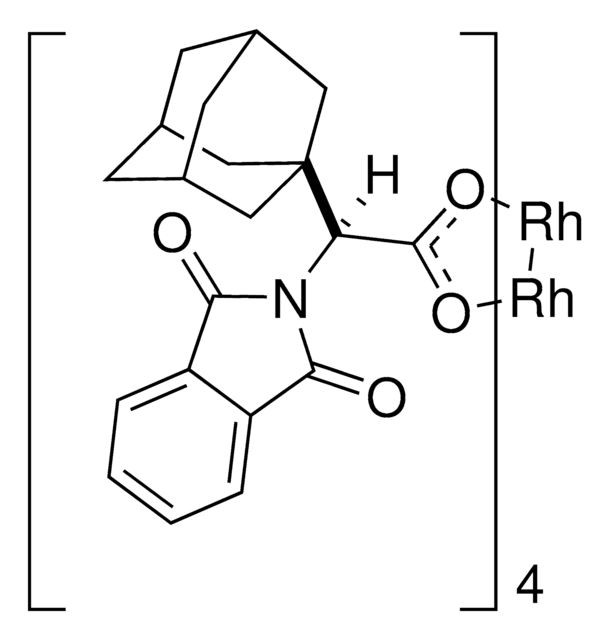



![双[(α,α,α′,α′-四甲基-1,3-苯二丙酸)铑] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)