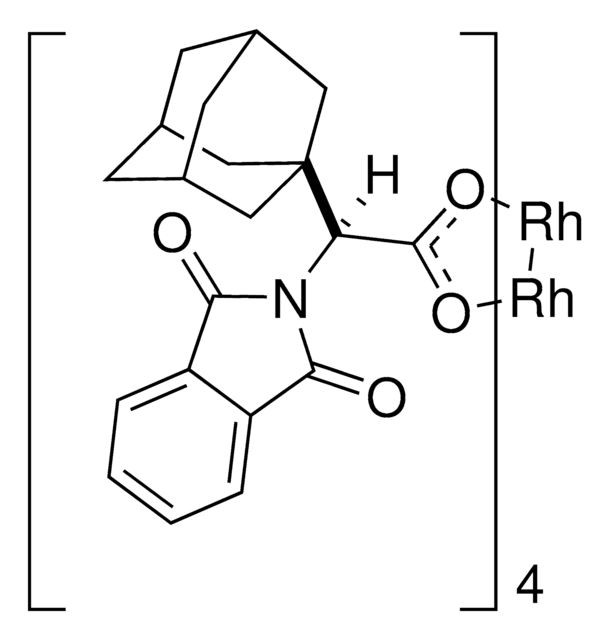
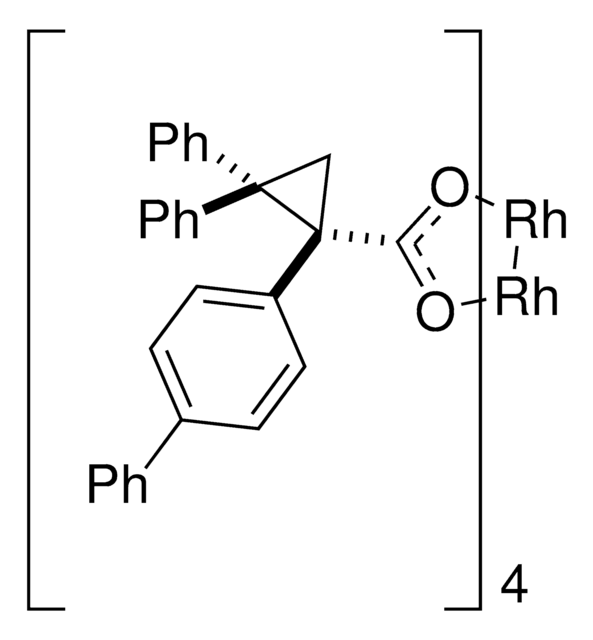
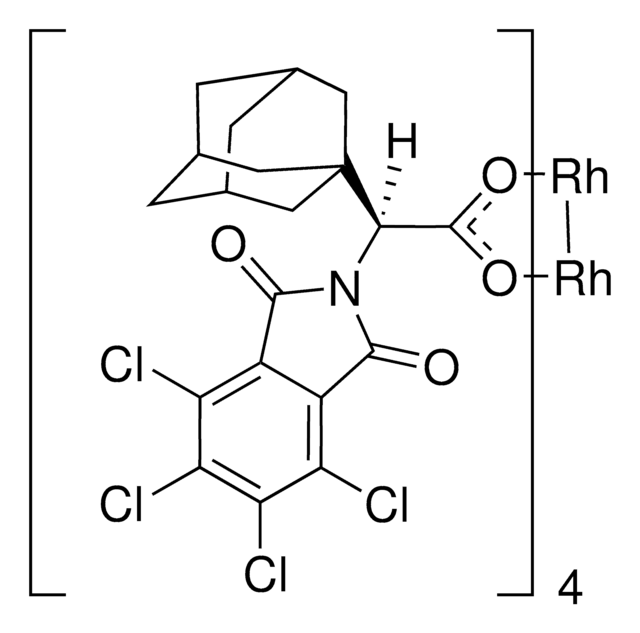
905291
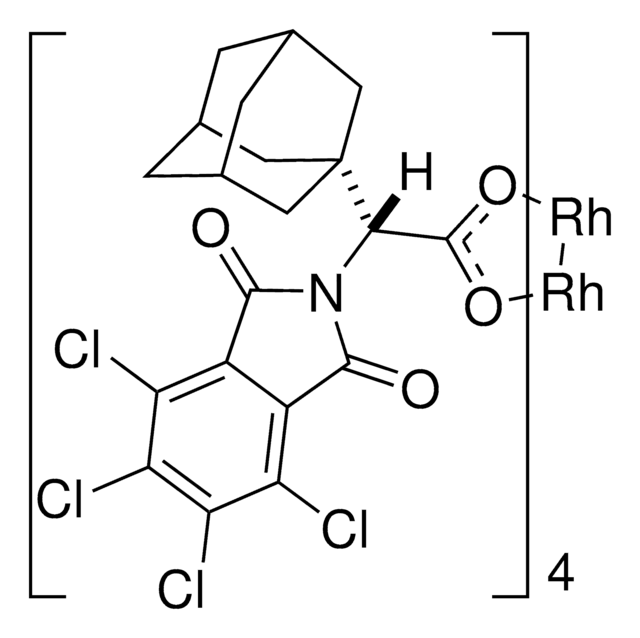
Rh2(R-BTPCP)4
别名:
Davies dirhodium catalyst, Dirhodium tetrakis((S,R)-1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylate), Dirhodium tetrakis[(R)-1-(4-bromophenyl)-2,2- diphenylcyclopropane carboxylate], Tetrakis[(R)-(-)-[(1R)-1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylato]dirhodium(II), Tetrakis[(R)-1-(4-bromophenyl)-2,2- diphenylcyclopropane carboxylato]dirhodium(II)
登录查看公司和协议定价
所有图片(3)
About This Item
推荐产品
形狀
powder or crystals
mp
>300 °C
相关类别
應用
Rh catalyst developed by the Davies lab used for enantioselective cyclopropanations and C-H funcionalization under low catalyst loadings.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Daniel Rackl et al.
Organic letters, 19(12), 3055-3058 (2017-06-06)
A tandem reaction system has been developed for the preparation of donor/acceptor-substituted diazo compounds in continuous flow coupled to dirhodium-catalyzed C-H functionalization or cyclopropanation. Hydrazones were oxidized in flow by solid-supported N-iodo-p-toluenesulfonamide potassium salt (PS-SO2NIK) to generate the diazo compounds
Changming Qin et al.
Journal of the American Chemical Society, 133(47), 19198-19204 (2011-11-04)
Dirhodium tetrakis-(R)-(1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylate) (Rh(2)(R-BTPCP)(4)) was found to be an effective chiral catalyst for enantioselective reactions of aryl- and styryldiazoacetates. Highly enantioselective cyclopropanations, tandem cyclopropanation/Cope rearrangements and a combined C-H functionalization/Cope rearrangement were achieved using Rh(2)(R-BTPCP)(4) as catalyst. The advantages of Rh(2)(R-BTPCP)(4)
Ji-Min Yang et al.
Journal of the American Chemical Society, 139(10), 3784-3789 (2017-02-15)
Herein, we report transition-metal-catalyzed B-H bond insertion reactions between borane adducts and alkynes to afford organoboron compounds in excellent yields under mild reaction conditions. This successful use of alkynes as carbene precursors in these reactions constitutes a new route to
Changming Qin et al.
Organic letters, 15(2), 310-313 (2013-01-05)
The rhodium-catalyzed reaction of 2-diazo-5-arylpent-4-enoates can be controlled by the appropriate choice of catalyst and catalyst loading to form either 2-arylbicyclo[1.1.0]butane carboxylates or cyclohexene derivatives. Both products are produced in a highly diastereoselective manner, with 2-arylbicyclo[1.1.0]butane carboxylates preferentially formed under
Kuangbiao Liao et al.
Nature, 551(7682), 609-613 (2017-11-21)
The synthesis of complex organic compounds usually relies on controlling the reactions of the functional groups. In recent years, it has become possible to carry out reactions directly on the C-H bonds, previously considered to be unreactive. One of the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![双[(α,α,α′,α′-四甲基-1,3-苯二丙酸)铑] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)