推荐产品
形狀
solid
儲存溫度
2-8°C
應用
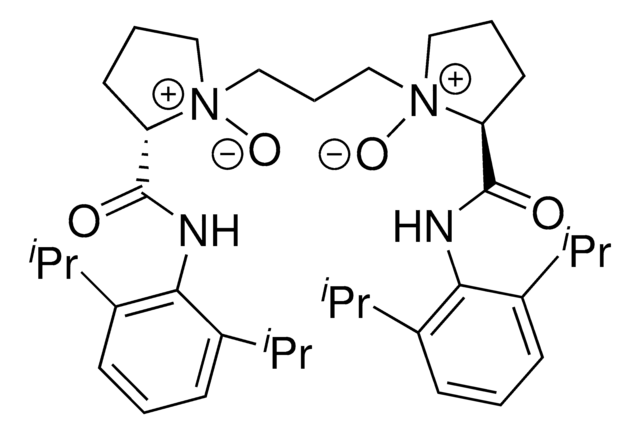
L3-PiPr2 is a chiral N,N-dioxide ligand developed by the Feng group. In conjunction with a variety of metal salts, this versatile ligand forms and active catalysts complex with application in many different reactions.
其他說明
An N,N′-Dioxide/In(OTf)3 Catalyst for the Asymmetric Hetero-Diels–Alder Reaction Between Danishefsky′s Dienes and Aldehydes: Application in the Total Synthesis of Triketide
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
An N,N'-dioxide/In(OTf)3 catalyst for the asymmetric Hetero-Diels-Alder reaction between Danishefsky's dienes and aldehydes: application in the total synthesis of triketide.
Zhipeng Yu et al.
Angewandte Chemie (International ed. in English), 47(7), 1308-1311 (2008-01-08)
Hang Zhang et al.
Chemical communications (Cambridge, England), 54(88), 12511-12514 (2018-10-23)
The catalytic asymmetric ene-type reactions of vinylogous hydrazone were accomplished by using chiral N,N'-dioxide-metal salt complexes as catalysts. A wide range of electrophiles, including isatins, α-ketoester, imines, and aldehydes reacted with (E)-2-methyl-N-(piperidin-1-yl)prop-2-en-1-imine efficiently, affording the corresponding homoallylic alcohols and amines
Xin Zhang et al.
The Journal of organic chemistry, 72(14), 5227-5233 (2007-06-15)
Complexes of (S)-pipecolic acid-, L-proline-, and other amino acid-derived N,N'-dioxides coordinated with different metal ions have been investigated in the enantioselective allylation of ketones. A variety of aromatic ketones were found to be suitable substrates in the presence of the
Xiaohu Zhao et al.
Angewandte Chemie (International ed. in English), 54(13), 4032-4035 (2015-02-05)
A highly efficient asymmetric dearomatization of indoles was realized through a cascade reaction between 2-isocyanoethylindole and alkylidene malonates catalyzed by a chiral N,N'-dioxide/Mg(II) catalyst. Fused polycyclic indolines containing three stereocenters were afforded in good yields with excellent diastereo- and enantioselectivities
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![N-[(1R,2R)-2-(1-哌啶基)环己基]-N′-[4-(三氟甲基)苯基]四酰胺 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)