推荐产品
一般說明
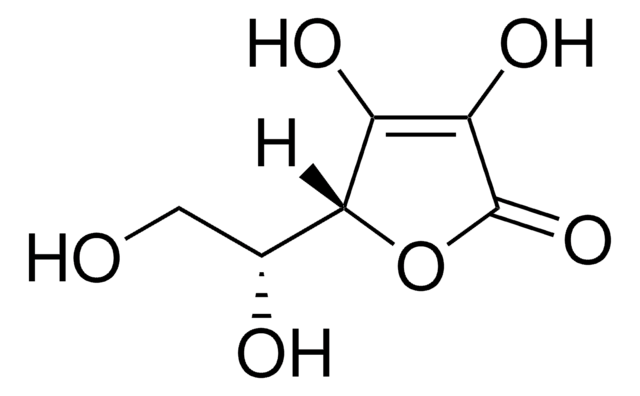
D-(−)-异抗坏血酸又名赤藻糖酸,广泛用作手性构件合成各种手性有机物。也用作各种有机反应的还原剂。
應用
D-(−)-异抗坏血酸可作为反应剂合成各种手性化合物,包括:
- 对映体纯氨基三醇
- (3R, 4S)-4-hydroxylasiodiplodin和D-mycinose
- α,β-二羟基-醛或酸的对映体纯立体异构体
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Andrew C Clark et al.
Journal of agricultural and food chemistry, 58(2), 1004-1011 (2009-12-31)
The stereochemical influence of antioxidant and flavanol compounds on oxidation processes in a model wine system was studied. The diastereoisomers, ascorbic acid and erythorbic acid, were used as antioxidants in a model wine system containing either (+)-catechin or (-)-epicatechin as
Manuel Bueno et al.
Carbohydrate research, 344(15), 2100-2104 (2009-07-17)
l-Ascorbic and d-isoascorbic acids have been used as the starting materials for the preparation of (3R,4'S)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTA), (3R and S, 4'S,6R)-3-methyl-6-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTP) and (3R,4'R)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPEA), three novel 1,4-dioxane-2,5-dione-type monomers. Ring-opening homopolymerisation and copolymerisation of the IPTA monomer, derived from l-ascorbic
Spyros Drivelos et al.
Analytical and bioanalytical chemistry, 397(6), 2199-2210 (2010-04-16)
A new hydrophilic interaction liquid chromatographic (HILIC) method for the simultaneous determination of isoascorbic (IAA) and ascorbic acid (AA) was developed. The separation of IAA and AA was studied in various HILIC stationary phases and the influence of the composition
Daiki Kyotani et al.
Bioscience, biotechnology, and biochemistry, 73(4), 954-956 (2009-04-09)
We found that a strain of Penicillium sp. effectively converted L-ascorbic acid to a five-carbon analog, which was identified as L-erythroascorbic acid based on spectroscopic analysis. The conversion was achieved by growing culture or washed mycelia, with a yield of
Alberto Baroja-Mazo et al.
Fungal genetics and biology : FG & B, 42(5), 390-402 (2005-04-06)
D-Erythroascorbate and D-erythroascorbate glucoside have been identified in the Zygomycete fungus Phycomyces blakesleeanus. Ascomycete and Basidiomycete fungi also synthesise D-erythroascorbate instead of l-ascorbate, suggesting that D-erythroascorbate synthesis evolved in the common ancestor of the fungi. Both compounds accumulate in P.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门