所有图片(1)
About This Item
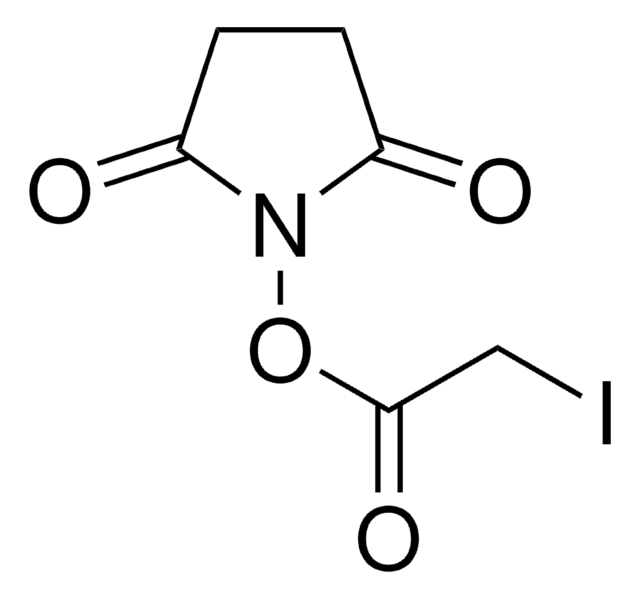
经验公式(希尔记法):
C9H11N2O5Br
CAS号:
分子量:
307.10
MDL號碼:
分類程式碼代碼:
12352106
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥90%
品質等級
形狀
powder
分子量
307.1
反應適用性
reagent type: cross-linking reagent
儲存條件
desiccated
溶解度
DMSO or DMF: soluble
官能基
NHS ester
bromo
運輸包裝
ambient
儲存溫度
2-8°C
SMILES 字串
O=C(CCC1=O)N1OC(CCNC(CBr)=O)=O
InChI
1S/C9H11BrN2O5/c10-5-6(13)11-4-3-9(16)17-12-7(14)1-2-8(12)15/h1-5H2,(H,11,13)
InChI 密鑰
WGMMKWFUXPMTRW-UHFFFAOYSA-N
一般說明
SBAP is a sulfhydryl-reactive and amine-reactive heterobifunctional crosslinker. The reagent′s NHS ester reacts with primary amines at pH 7-9 to form stable amide bonds, and the bromacetyl reacts with sulfhydryl groups at pH >7.5 to form stable thioether bonds This reagent is useful for preparing cyclic peptides and peptide conjugates because the spacer maintains peptide-like character in the crosslinked species.
特點和優勢
- Reactive groups: NHS ester and bromoacetyl
- Reactive toward: amino and sulfhydryl groups
- NHS ester reacts with primary amines at pH 7-9 to form a stable amide bond
- Bromacetyl group reacts with sulfhydryl groups at pH > 7.5 to form stable thioether bonds
- Non-cleavable
- Water-insoluble (dissolve first in DMF or DMSO)
- Spacer maintains peptide-like character in the crosslinked species
- Resulting crosslink is susceptible to acid hydrolysis
- Useful for preparing cyclic peptides and peptide conjugates
注意
This product is sensitive to moisture. The vial is packaged in a resealable bag with a desiccant to reduce exposure to moisture. After cold storage, equilibrate the vial to room temperature before opening to reduce condensation inside the vial. Make fresh solutions. Storage of stock solutions is not recommended. After use, return the vial to the resealable bag. Close the bag and store the product at the recommended temperature.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
J K Inman et al.
Bioconjugate chemistry, 2(6), 458-463 (1991-11-01)
A new amino acid derivative, N alpha-(tert-butoxycarbonyl)-N epsilon-[N-(bromoacetyl)-beta-alanyl]-L-lysine (BBAL), has been synthesized as a reagent to be used in solid-phase peptide synthesis for introducing a side-chain bromoacetyl group at any desired position in a peptide sequence. The bromoacetyl group subsequently
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门