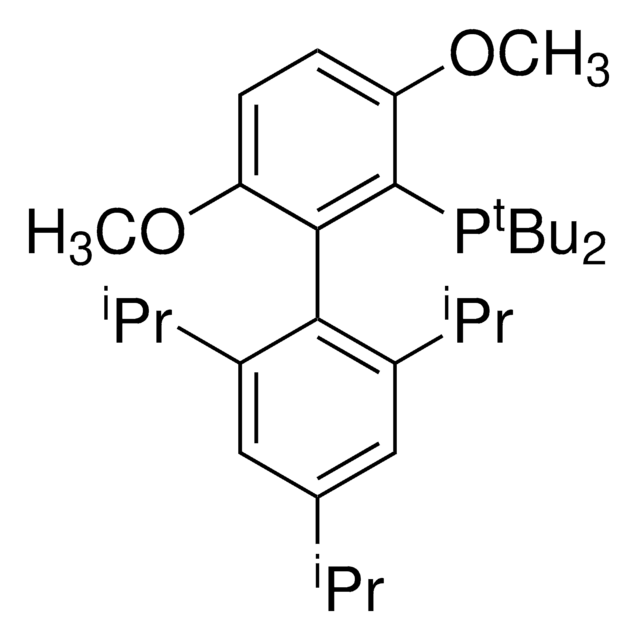
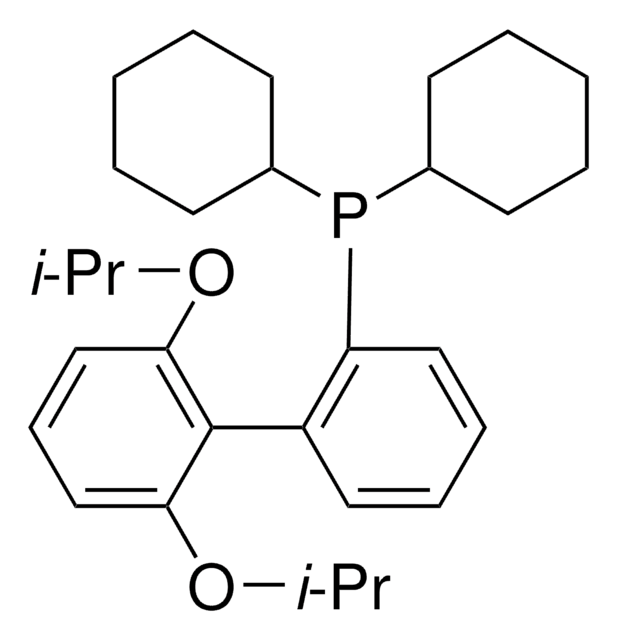
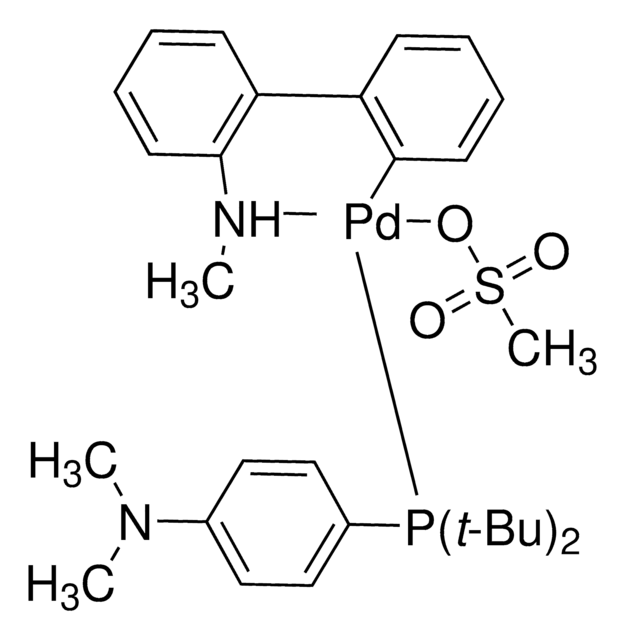
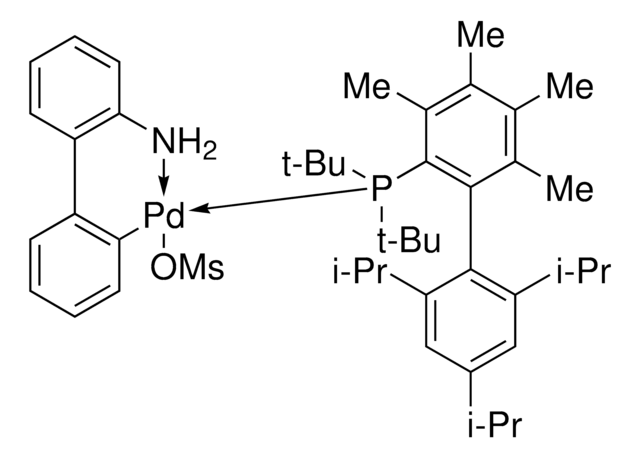
推荐产品
品質等級
化驗
97%
形狀
solid
特點
generation 1
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
198-204 °C
官能基
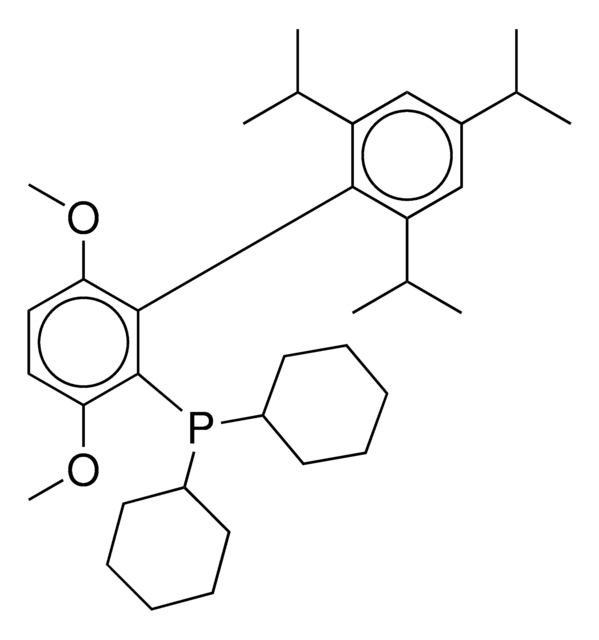
phosphine
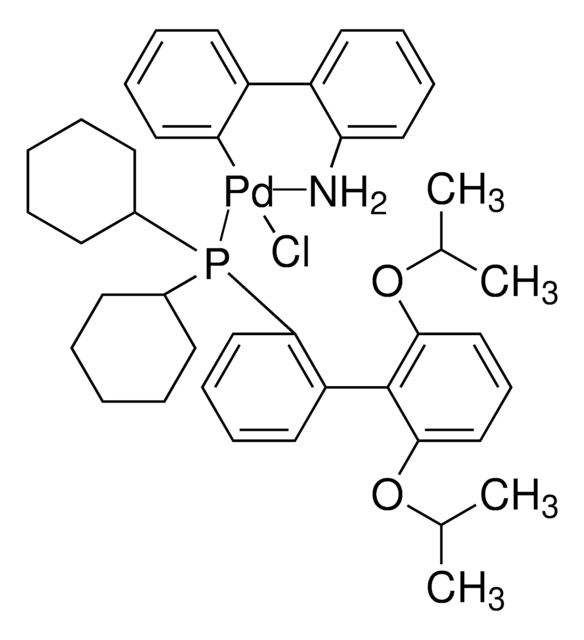
SMILES 字串
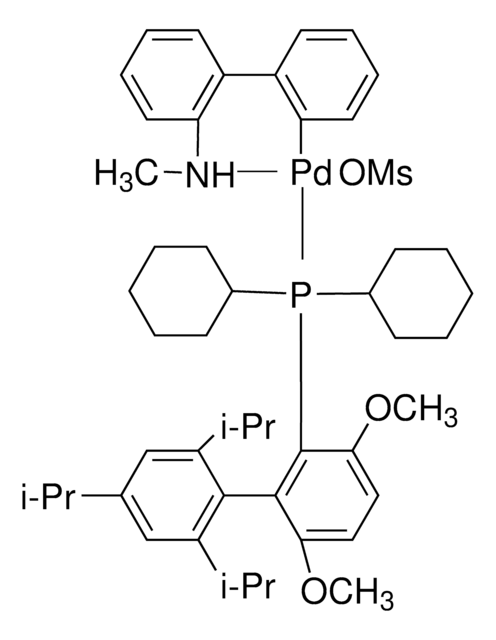
COC(C)(C)C.NCCc1ccccc1[Pd]Cl.COc2ccc(OC)c(c2P(C3CCCCC3)C4CCCCC4)-c5c(cc(cc5C(C)C)C(C)C)C(C)C
InChI
1S/C35H53O2P.C8H10N.C5H12O.ClH.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;9-7-6-8-4-2-1-3-5-8;1-5(2,3)6-4;;/h19-25,27-28H,9-18H2,1-8H3;1-4H,6-7,9H2;1-4H3;1H;/q;;;;+1/p-1
InChI 密鑰
OWHWOTGYDWMPCA-UHFFFAOYSA-M
一般說明
應用
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 718750-5G | 4061832855219 |
| 718750-100MG | 4061833229071 |
| 718750-1G | 4061826644508 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持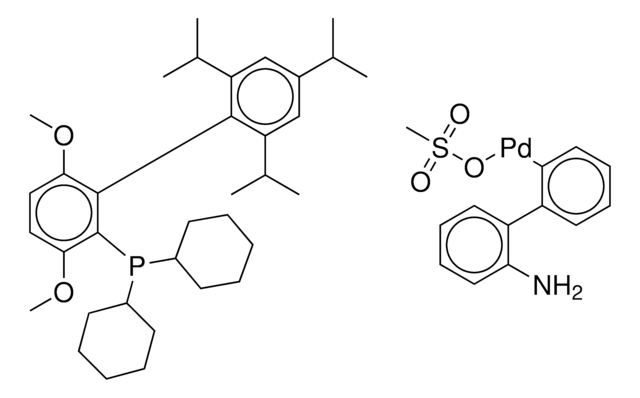

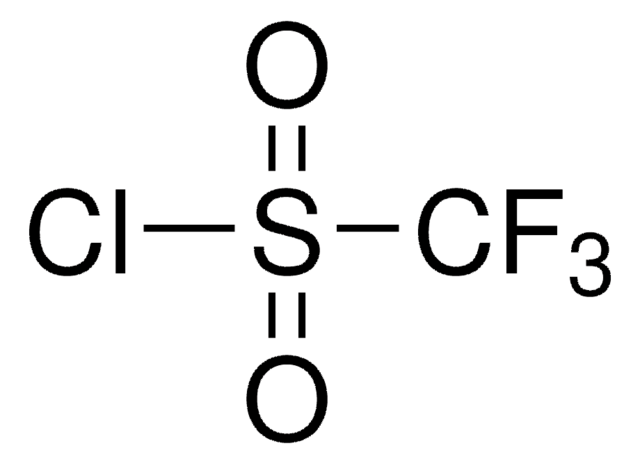
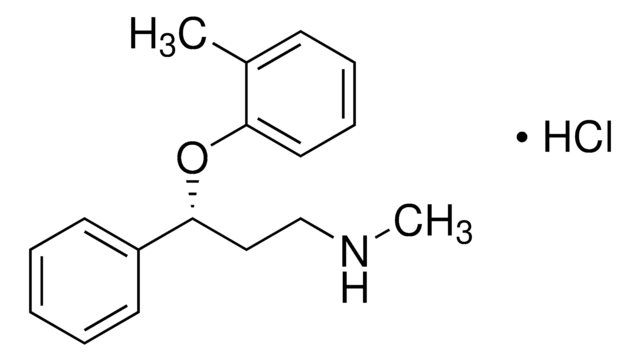
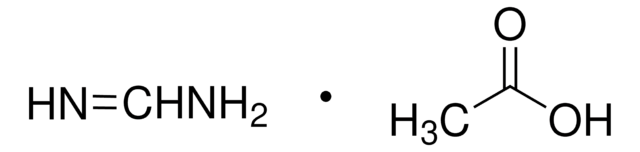
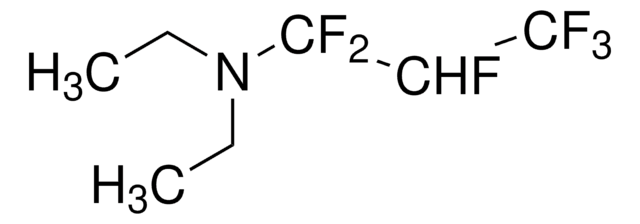
 95%](/deepweb/assets/sigmaaldrich/product/structures/151/609/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1/640/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1.png)