About This Item
推荐产品
品質等級
化驗
95%
形狀
solid
光學活性
[α]20/D -134°, c = 1 in methanol
mp
136-142 °C
官能基
amide
phosphine
SMILES 字串
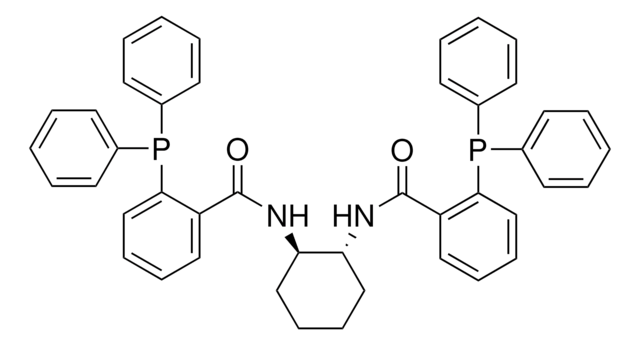
O=C(N[C@H]1CCCC[C@@H]1NC(=O)c2ccccc2P(c3ccccc3)c4ccccc4)c5ccccc5P(c6ccccc6)c7ccccc7
InChI
1S/C44H40N2O2P2/c47-43(37-27-13-17-31-41(37)49(33-19-5-1-6-20-33)34-21-7-2-8-22-34)45-39-29-15-16-30-40(39)46-44(48)38-28-14-18-32-42(38)50(35-23-9-3-10-24-35)36-25-11-4-12-26-36/h1-14,17-28,31-32,39-40H,15-16,29-30H2,(H,45,47)(H,46,48)/t39-,40-/m0/s1
InChI 密鑰
AXMSEDAJMGFTLR-ZAQUEYBZSA-N
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
商品
Palladium-catalyzed asymmetric allylic alkylation (AAA) has proven to be an exceptionally powerful method for the efficient construction of stereogenic centers.
The Trost group at Stanford University has pioneered the use of C-2 symmetric diaminocyclohexyl (DACH) ligands in AAA, allowing for the rapid synthesis of a diverse range of chiral products with a limited number of chemical transformations.
The Trost group at Stanford University has pioneered the use of C-2 symmetric diaminocyclohexyl (DACH) ligands in AAA, allowing for the rapid synthesis of a diverse range of chiral products with a limited number of chemical transformations.
Palladium-catalyzed asymmetric allylic alkylation (AAA) has proven to be an exceptionally powerful method for the efficient construction of stereogenic centers.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门