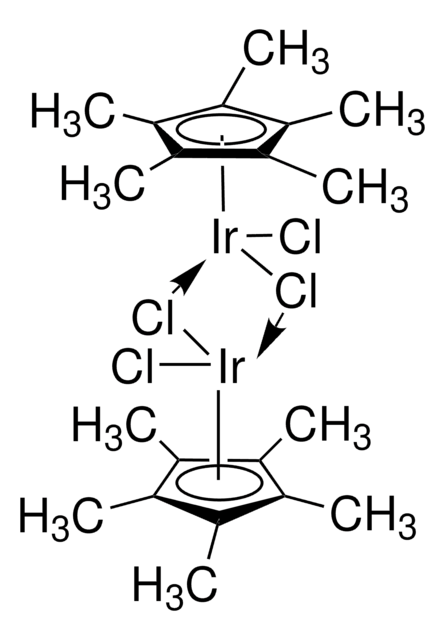
About This Item
推荐产品
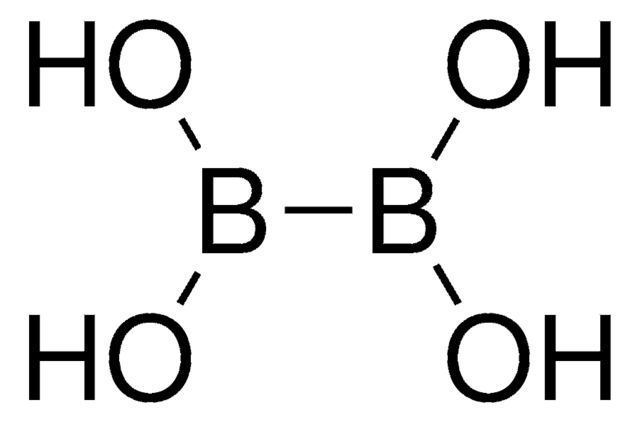
形狀
crystals
品質等級
反應適用性
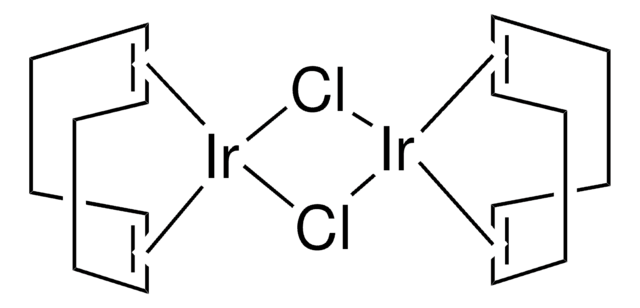
core: iridium
reagent type: catalyst
reaction type: C-H Activation
mp
154-179 °C (D)
儲存溫度
−20°C
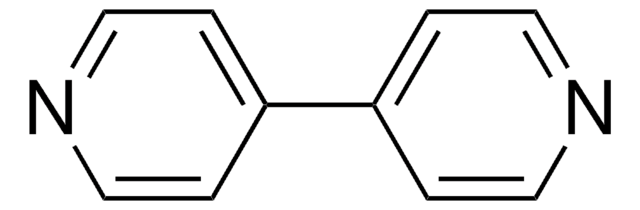
SMILES 字串
C[O+]1[Ir-]2[O+](C)[Ir-]12.C3CC=CCCC=C3.C4CC=CCCC=C4
InChI
1S/2C8H12.2CH3O.2Ir/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*+1;2*-1/b2*2-1-,8-7-;;;;
InChI 密鑰
BGWIAAATAAWGOI-MIXQCLKLSA-N
應用
- 制备杂芳基稠合的吲哚环体系,作为HCV NS5B聚合酶的抑制剂
- 硼化反应/Suzuki-Miyaura偶联反应
- Metalation-Suzuki交叉偶联反应,用于合成联芳基和杂双芳基
- 四硼化反应
- 高度区域和对映选择性不对称硼氢化
- 通过C-H活化使芳基酮、苯甲醛和苄醇衍生物发生邻甲硅烷基化
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)