所有图片(3)
About This Item
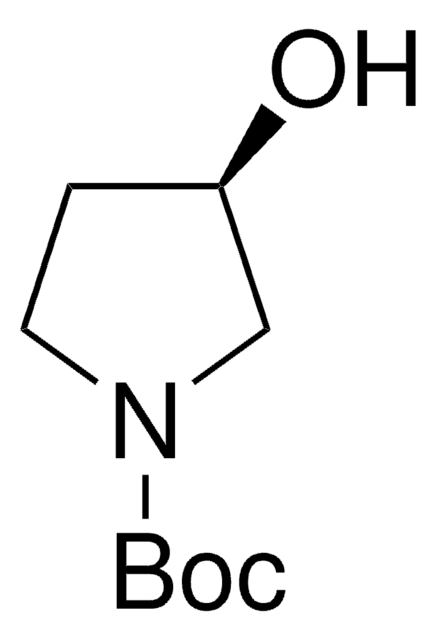
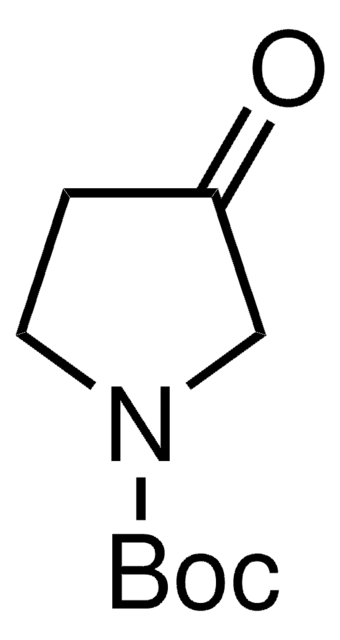
经验公式(希尔记法):
C9H17NO3
CAS号:
分子量:
187.24
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
光學活性
[α]20/D +26°, c = 1% in methanol
mp
60-64 °C (lit.)
官能基
hydroxyl
SMILES 字串
CC(C)(C)OC(=O)N1CC[C@H](O)C1
InChI
1S/C9H17NO3/c1-9(2,3)13-8(12)10-5-4-7(11)6-10/h7,11H,4-6H2,1-3H3/t7-/m0/s1
InChI 密鑰
APCBTRDHCDOPNY-ZETCQYMHSA-N
應用
(S)-(+)-N-Boc-3-pyrrolidinol can be used as a reactant to synthesize:
- tert-Butyl 3-(2-bromophenoxy)pyrrolidine-1-carboxylate, which is employed as a key intermediate in the preparation of IkB-kinase IKK2 inhibitor.
- Dimethoxy-pyrrolidylquinazoline , and peptidomimetic quinoline derivatives.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Discovery of selective irreversible inhibitors for Bruton?s tyrosine kinase
P Zhengying, et al.
ChemMedChem, 2(1), 58-61 (2007)
Exploiting the Differential Reactivities of Halogen Atoms: Development of a Scalable Route to IKK2 Inhibitor AZD3264
Murugan A, et al.
Organic Process Research & Development, 18(5), 646-651 (2014)
Erik A A Wallén et al.
Journal of medicinal chemistry, 46(21), 4543-4551 (2003-10-03)
Isophthalic acid bis(l-prolyl-pyrrolidine) amide is a very potent prolyl oligopeptidase inhibitor, but it has a log P value of -0.2, which is very low for a compound targeted to the brain. Therefore, these types of compounds were further modified to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门