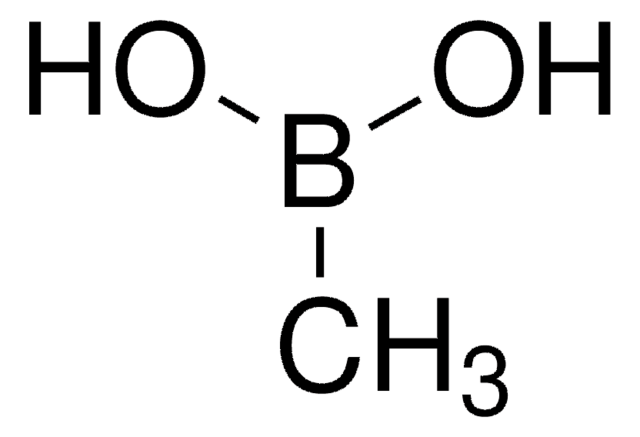
About This Item
推荐产品
化驗
98%
折射率
n20/D 1.506 (lit.)
bp
282 °C (lit.)
密度
1.05 g/mL at 25 °C (lit.)
SMILES 字串
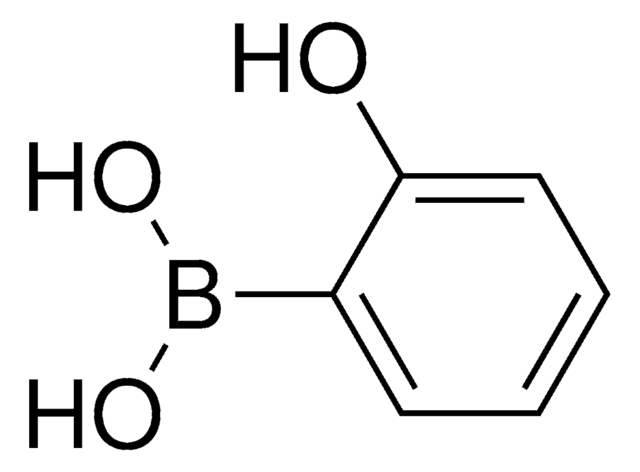
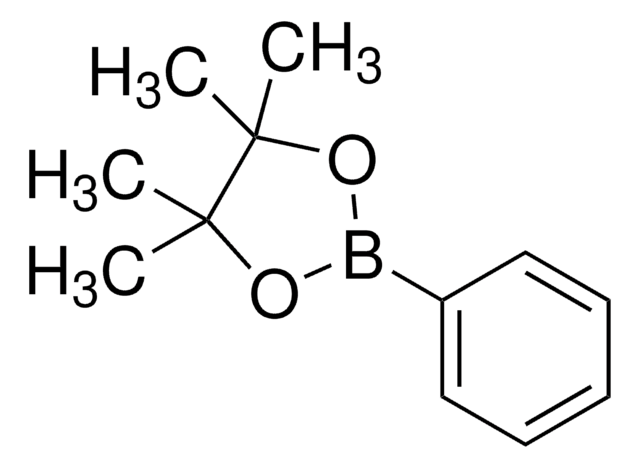
CC1(C)OB(OC1(C)C)c2ccccc2O
InChI
1S/C12H17BO3/c1-11(2)12(3,4)16-13(15-11)9-7-5-6-8-10(9)14/h5-8,14H,1-4H3
InChI 密鑰
VLROJECCXBBKPZ-UHFFFAOYSA-N
應用
- Synthesis of indolo-fused heterocyclic inhibitors of polymerase enzyme of hepatitis C
- Studies of pi-interactions, electronic structure and transient UV absorption of subphthalocyanine-borate-bridged ferrocene-fullerene conjugates
- Synthesis of subphthalocyanine and fused-ring nicotine derivatives
- Suzuki-Miyaura coupling-triflation sequence, reduction and salt formation for synthesis of hydroxylated oligoarene phosphines
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
商品
The synthesis of biaryl compounds via the Suzuki coupling reaction has become more commonplace now that many arylboronic acids are readily available. We are pleased to offer arylboronic acid pinacol esters4 as part of a growing line of products used in the Suzuki coupling reaction.
The synthesis of biaryl compounds via the Suzuki coupling reaction has become more commonplace now that many arylboronic acids are readily available. We are pleased to offer arylboronic acid pinacol esters4 as part of a growing line of products used in the Suzuki coupling reaction.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门