所有图片(1)
About This Item
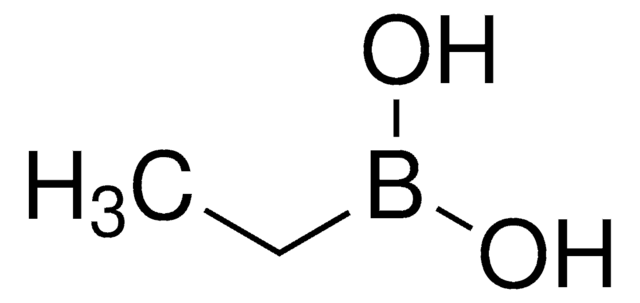
线性分子式:
CH3B(OH)2
CAS号:
分子量:
59.86
Beilstein:
1731087
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
形狀
solid
mp
91-94 °C (lit.)
SMILES 字串
CB(O)O
InChI
1S/CH5BO2/c1-2(3)4/h3-4H,1H3
InChI 密鑰
KTMKRRPZPWUYKK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
甲基硼酸可用作以下反应中的试剂:
- 钯催化的 Stille 和 Suzuki-Miyaura 交叉偶联。
- 微波加热的非均相钯(Pd)催化反应。
- 钌(Ru)催化甲硅烷基化反应
- 制备双(氨基丙酮)钛(Ti)催化剂,用于乙烯聚合反应。
- 使用氨基硫代氨基甲酸酯催化剂催化选择性不对称溴氨基环化和溴氨基环化。
- 制备药品和农用化学品的基础成分。
- 通过 Suzuki-Miyaura 偶联反应制备白杨类似物。
- 制备酪蛋白激酶 I 抑制剂。
- 在药物研发中,制备由磺胺药物药效团引导的不同 C-H 官能化。
- 通过铜催化与硼酸偶联,从二硫化物合成不对称一硫化物。
- 钯催化与烯醇酯偶联。
- 衍生用于GLC分析的多种碳水化合物和生物活性化合物。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Dietrich Steinhuebel et al.
The Journal of organic chemistry, 70(24), 10124-10127 (2005-11-19)
[reaction: see text] Herein we demonstrate functionalized enol tosylates to be robust substrates that undergo Suzuki-Miyaura, Sonogashira, and Stille cross-coupling reactions to provide stereodefined trisubstituted unsaturated esters.
Ben W Glasspoole et al.
Chemical communications (Cambridge, England), 48(9), 1230-1232 (2011-12-20)
Palladium-catalyzed cross-coupling reactions of secondary allylic boronic esters with iodoarenes were demonstrated under the conditions previously described for the coupling of benzylic substrates. The regioselectivity of the process was largely dictated by the pattern of olefin substitution.
A comparative study of ethylene polymerization by bis(aminotropone) Ti catalysts
Goldani, M. T.; et al.
Polym. Bull., 68, 755-773 (2012)
Ling Zhou et al.
Journal of the American Chemical Society, 132(44), 15474-15476 (2010-10-16)
A novel amino-thiocarbamate-catalyzed bromolactonization of unsaturated carboxylic acids has been developed. The scope of the reaction is evidenced by 22 examples of γ-lactones with up to 99% yield and 93% ee. The protocol was applied in the enantioselective synthesis of
Ling Zhou et al.
Journal of the American Chemical Society, 133(24), 9164-9167 (2011-05-05)
A facile and efficient enantioselective bromoaminocyclization of unsaturated sulfonamides has been developed using an amino-thiocarbamate catalyst. A range of enantioenriched pyrrolidines were prepared with up to 99% yield and 99% ee. The corresponding lactams could be obtained through oxidation of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)