所有图片(2)
About This Item
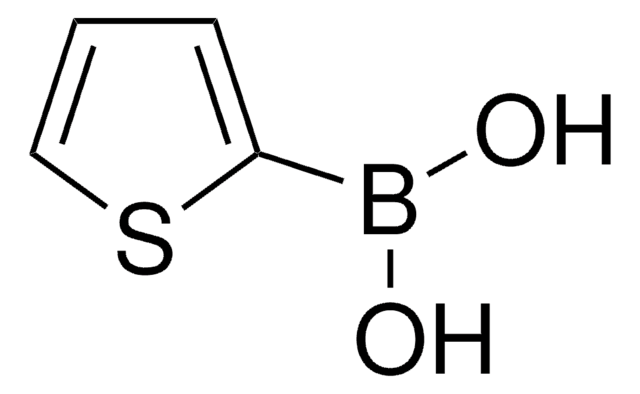
经验公式(希尔记法):
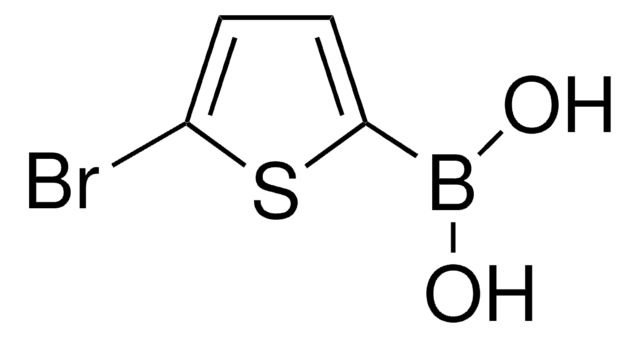
C8H7BO2S
CAS号:
分子量:
178.02
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥95%
mp
256-260 °C (lit.)
SMILES 字串
OB(O)c1cc2ccccc2s1
InChI
1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H
InChI 密鑰
YNCYPMUJDDXIRH-UHFFFAOYSA-N
應用
Reactant involved in:
- PDE4 inhibitors
- Chemoselective modification of oncolytic adenovirus
- Synthesis of phosphorescent sensor for quantification of copper(II) ion
- UV promoted phenanthridine syntheses
- Preparation of CYP11B1 inhibitors for treatment of cortisol dependent diseases
- Suzuki-Miyaura cross-coupling reactions
其他說明
含不定量的酸酐
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Hyomin Jin et al.
Dalton transactions (Cambridge, England : 2003), 48(4), 1467-1476 (2019-01-12)
2-Phenylpyridine- and 2-(benzo[b]thiophen-2-yl)pyridine-based (ppy- and btp-based) o-carboranyl (Car1 and Car2) and their B(CH3)2-C∧N-chelated (Car1B and Car2B) compounds were prepared and fully characterised by multinuclear NMR spectroscopy and elemental analysis. The solid-state structure of Car2B was determined by single-crystal X-ray diffraction
S A Adediran et al.
Archives of biochemistry and biophysics, 614, 65-71 (2017-01-01)
O-Aryloxycarbonyl hydroxamates have previously been shown to covalently inactivate serine/amine amidohydrolases such as class C β-lactamases and a N-terminal hydrolase, the proteasome. We report here reactions between O-aryloxycarbonyl hydroxamates and another N-terminal hydrolase, penicillin acylase. O-Aryloxycarbonyl hydroxamates, as non-symmetric carbonates
Ramona Iseppi et al.
Microbial drug resistance (Larchmont, N.Y.), 24(8), 1156-1164 (2018-02-17)
We investigated the occurrence of extended-spectrum β-lactamase (ESBL), AmpC, and carbapenemase-producing Gram-negative bacteria isolated from 160 samples of fresh vegetables (n = 80) and ready-to-eat (RTE) prepacked salads (n = 80). Phenotypic and genotypic analyses were carried out on the isolates in terms of
Hyomin Jin et al.
Molecules (Basel, Switzerland), 24(1) (2019-01-10)
Herein, we investigated the effect of ring planarity by fully characterizing four pyridine-based o-carboranyl compounds. o-Carborane was introduced to the C4 position of the pyridine rings of 2-phenylpyridine and 2-(benzo[b]thiophen-2-yl)pyridine (CB1 and CB2, respectively), and the compounds were subsequently borylated
Alberto Venturelli et al.
Journal of medicinal chemistry, 50(23), 5644-5654 (2007-10-25)
Benzo[b]thiophene-2-ylboronic acid, 1, is a 27 nM inhibitor of the class C beta-lactamase AmpC and potentiates the activity of beta-lactam antibiotics in bacteria that express this and related enzymes. As is often true, the potency of compound 1 against the
商品
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![苯并[b]噻吩并-3-基硼酸 ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/136/961/9ddc053e-3519-47d3-be03-95715d131635/640/9ddc053e-3519-47d3-be03-95715d131635.png)