About This Item
推荐产品
品質等級
化驗
96%
折射率
n20/D 1.453 (lit.)
bp
129-130 °C (lit.)
密度
1.01 g/mL at 25 °C (lit.)
官能基
amine
chloro
儲存溫度
2-8°C
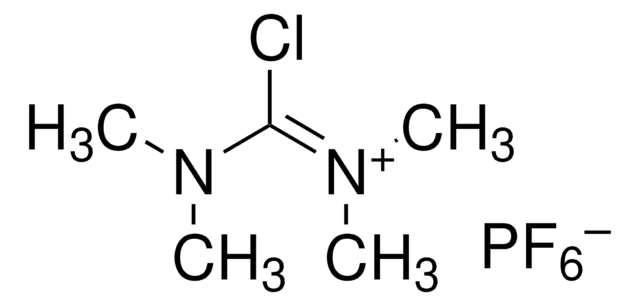
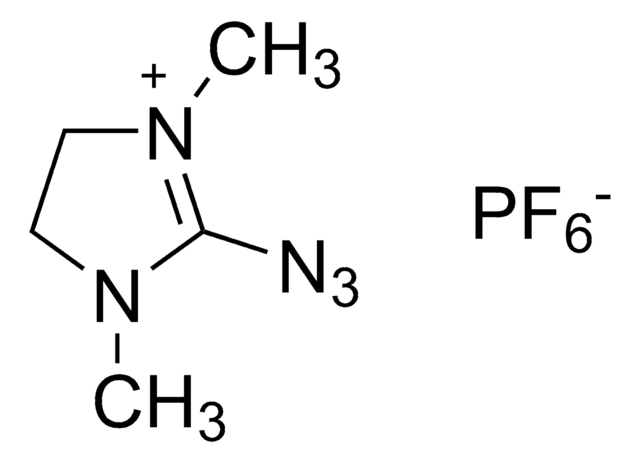
SMILES 字串
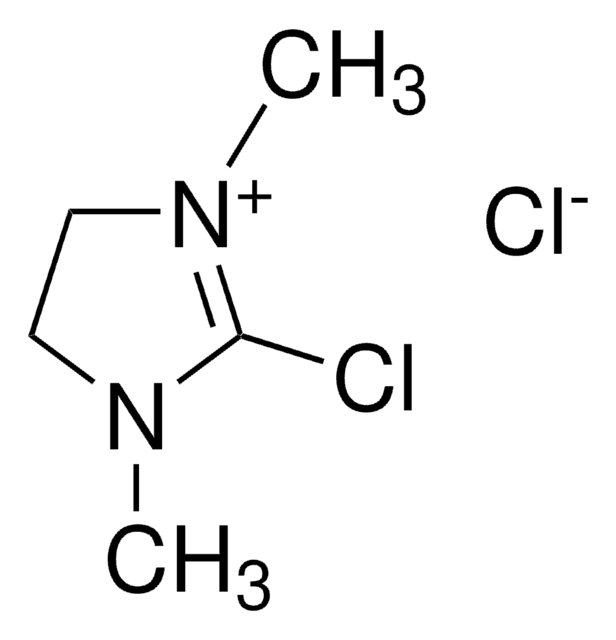
CN(C)\C(Cl)=C(\C)C
InChI
1S/C6H12ClN/c1-5(2)6(7)8(3)4/h1-4H3
InChI 密鑰
GQIRIWDEZSKOCN-UHFFFAOYSA-N
應用
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 3 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
80.0 °F - closed cup
閃點(°C)
26.67 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
商品
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持