推荐产品
蒸汽密度
3.6 (vs air)
品質等級
蒸汽壓力
60 mmHg ( 25 °C)
等級
absolute
化驗
≥99.0% (GC)
形狀
liquid
自燃溫度
346 °F
品質
over molecular sieve (H2O ≤0.01%)
折射率
n20/D 1.373 (lit.)
n20/D 1.374
bp
87-88 °C (lit.)
密度
0.831 g/mL at 25 °C (lit.)
SMILES 字串
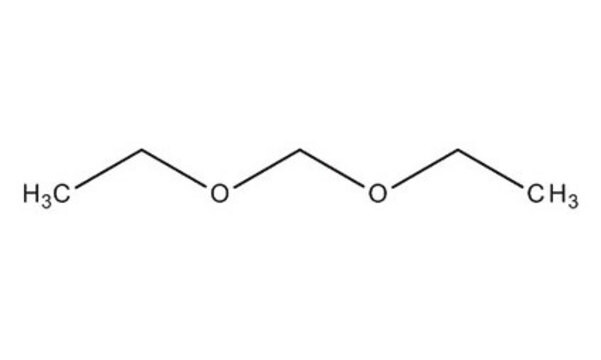
CCOCOCC
InChI
1S/C5H12O2/c1-3-6-5-7-4-2/h3-5H2,1-2H3
InChI 密鑰
KLKFAASOGCDTDT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Formaldehyde diethyl acetal (FDEA, diethoxymethane, DEM) is a diethyl acetal. It has been synthesized from ethanol and aqueous formaldehyde. It is a low boiling solvent useful for sodium hydride reactions, organolithium chemistry, copper-catalyzed conjugate additions and phase-transfer reactions. It is a potential alternate for tetrahydrofuran, dichloromethane, glyme and methylal. DEM is an ethoxymethylating agent, a formaldehyde equivalent and a carbonylation substrate. Its condensation with 2-propylresorcinol to form resorcin[n]arenes (n=4–7) has been investigated. The IR and Raman spectra and conformations of DEM have been studied. NMR spectroscopy has been used to study the aggregation behavior of organolithium compounds in diethoxymethane. Unimolecular metastable decomposition of DEM upon electron impact has been studied. Solubility of non-polar gases in DEM has been evaluated.
應用
Formaldehyde diethyl acetal may be used as a solvent in the esterification of carboxylic acids and in the O-alkylation of a variety of phenols.
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
23.0 °F - closed cup
閃點(°C)
-5 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Metastable decompositions of gem-dialkoxyalkanes upon electron impact. III. Diethoxymethane (CH2 (OCH2CH3)2).
Tajima S, et al.
Rapid Communications in Mass Spectrometry, 14(14), 1195-1199 (2000)
Study on the synthesis of diethoxy methane using toluene-p-sulfonic acid as catalyst.
Gu Z, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 22(12), 1368-1368 (2005)
Solubility of non polar gases in formaldehyde diethyl acetal between-10 and 30?C, and 1 Atm partial pressure of gas.
Lizano LP, et al.
Journal of Solution Chemistry, 19(7), 721-728 (1990)
Use of Diethoxymethane as a Solvent for Phase-Transfer Esterification of Carboxylic Acids.
Coleman MT.
Synthetic Communications, 42(13), 1911-1913 (2012)
NMR spectroscopy of organolithium compounds, Part XVI: The aggregation behavior of butyllithium, phenyllithium, and lithium diisopropylamide in dimethoxy-and diethoxymethane.
Bergander K, et al.
Tetrahedron, 50(20), 5861-5868 (1994)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门