所有图片(1)
About This Item
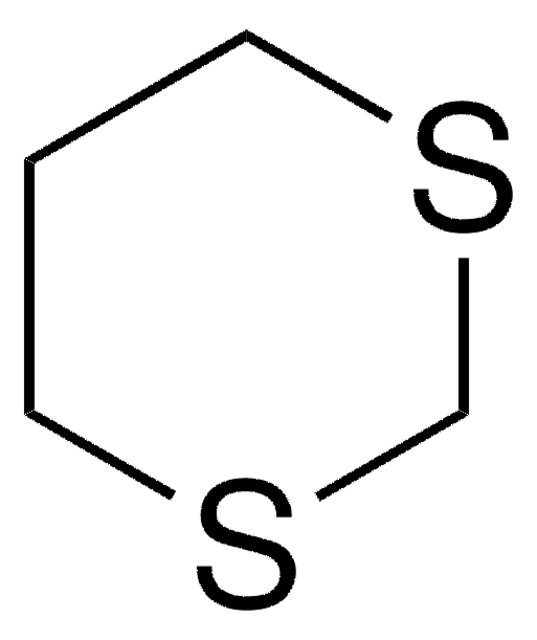
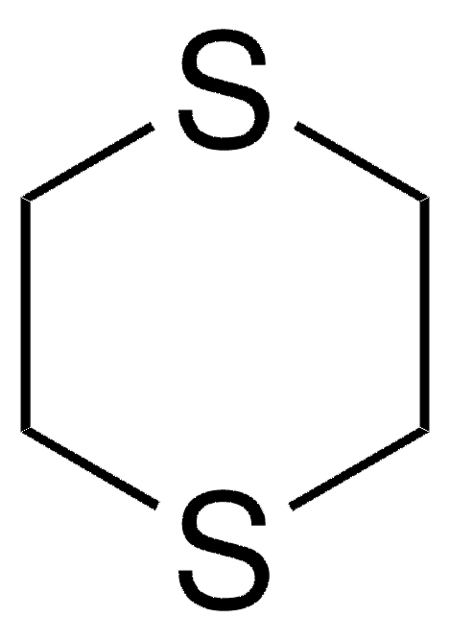
经验公式(希尔记法):
C3H6S2
CAS号:
分子量:
106.21
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
折射率
n20/D 1.599 (lit.)
bp
183 °C (lit.)
密度
1.235 g/mL at 25 °C (lit.)
官能基
thioether
SMILES 字串
C1CSCS1
InChI
1S/C3H6S2/c1-2-5-3-4-1/h1-3H2
InChI 密鑰
IMLSAISZLJGWPP-UHFFFAOYSA-N
一般說明
1,3-Dithiolane ,a sulfur containing heterocyclic building block, is a cyclic thioether. Fragmentation modes of 1,3-oxathiolane under electron-impact have been investigated.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
154.4 °F - closed cup
閃點(°C)
68 °C - closed cup
Sylvie Goncalves et al.
Chemical communications (Cambridge, England), 46(31), 5778-5780 (2010-07-06)
A concise and diastereoselective formal total synthesis of triptolide, a natural product with a wide range of biological properties, is described. The key reaction is an unprecedented 6-endo-Trig cationic cyclization of a 2-alkenyl-1,3-dithiolane precursor induced by TMSOTf as Lewis acid.
Alexandra Papanikos et al.
Journal of combinatorial chemistry, 6(2), 181-195 (2004-03-09)
A synthetic strategy for the formation of resin-bound internal alpha-keto amide peptides suitable for protease inhibitor screening on solid support is presented. This general approach is based on the incorporation of alpha-keto amide building blocks during solid-phase peptide synthesis (SPPS).
I M Hussaini et al.
Acta pharmacologica Sinica, 21(10), 897-904 (2001-08-15)
To investigate the effect of a group of novel synthetic dithiolane analogs of lignans and a well characterized platelet-activating factor (PAF) receptor antagonist, L659,989 on PAF-receptor binding, IFN-gamma- and lipopolysaccharide (LPS)-induced NO production, and steady-state inducible nitric-oxide synthase (iNOS) mRNA
Kalevi Pihlaja et al.
Magnetic resonance in chemistry : MRC, 49(7), 443-449 (2011-05-07)
1-Oxo-1,3-dithiolane (4) and its cis- and trans-2-methyl (5,6), -4-methyl (7,8) and -5-methyl (9,10) derivatives were prepared by oxidizing the corresponding 1,3-dithiolanes (1-3) with NaIO(4) in water. The oxides were purified and their isomers separated using thin layer chromatography. The structural
Ionization and dissociation of cyclic ethers and thioethers by electron-impact. A comparison between 1, 3-dioxolane, 1, 3-dithiolane and 1, 3-oxathiolane.
Conde-Caprace G and Collin JE.
Org. Mass Spectrom., 6(4), 415-423 (1972)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门