所有图片(1)
About This Item
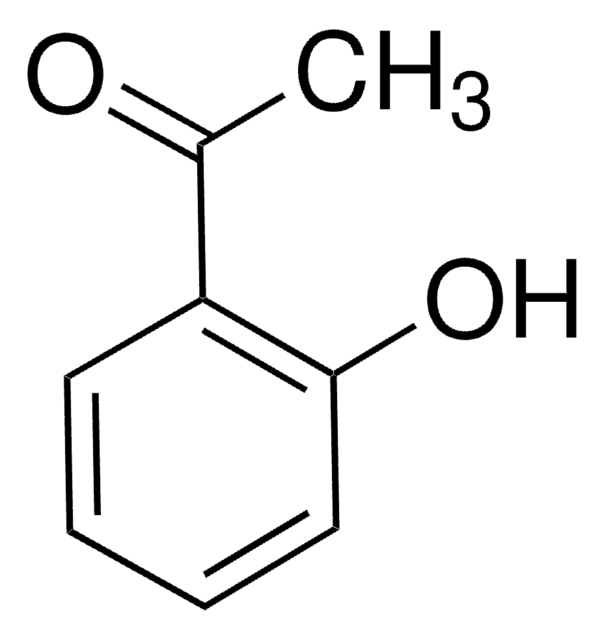
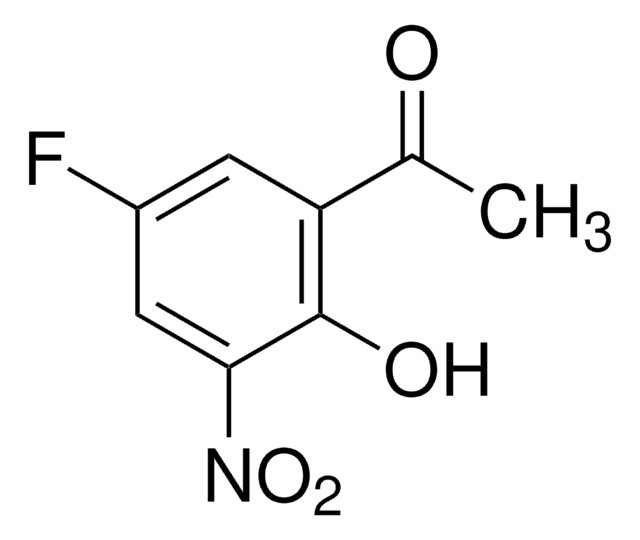
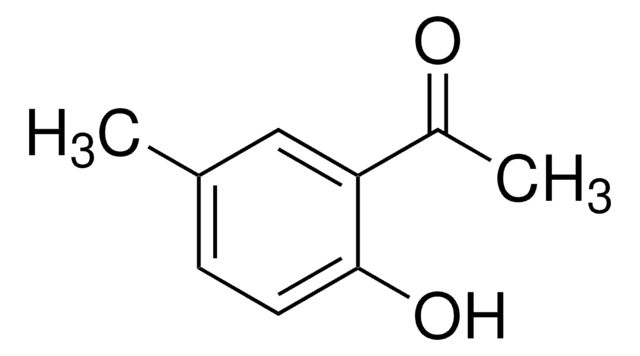
线性分子式:
FC6H3(OH)COCH3
CAS号:
分子量:
154.14
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
mp
31-35 °C (lit.)
官能基
fluoro
ketone
SMILES 字串
CC(=O)c1ccc(F)cc1O
InChI
1S/C8H7FO2/c1-5(10)7-3-2-6(9)4-8(7)11/h2-4,11H,1H3
InChI 密鑰
HLTBTUXAMVOKIH-UHFFFAOYSA-N
一般說明
4′-Fluoro-2′-hydroxyacetophenone is a substituted acetophenone derivative. Biological Baeyer-Villiger oxidation of 4′-fluoro-2′-hydroxyacetophenone to 4-fluorocatechol by using whole cells of Pseudomonas fluorescens ACB has been reported. Its crystals belong to the monoclinic crystal system and space group P21/n.
應用
最近用于制备具有药用活性的苯并[b]呋喃类化合物和噻吩类化合物。
4′-Fluoro-2′-hydroxyacetophenone may be used in the preparation of series of 4′-fluoro-2′-hydroxychalcones, via aldol condensation with substituted aldehydes followed by cyclization with hydrazine hydrate.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
210.2 °F - closed cup
閃點(°C)
99 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
4'-Fluoro-2'-hydroxyacetophenone.
Rizal MR and Ng SW.
Acta Crystallographica Section E, Structure Reports Online, 64(5), o916-o916 (2008)
Khaled R A Abdellatif et al.
Journal of enzyme inhibition and medicinal chemistry, 30(3), 484-491 (2014-09-10)
In an effort to develop safe and potent anti-inflammatory agents, a series of novel 4'-fluoro-2'-hydroxychalcones 5a-d and their dihydropyrazole derivatives 6a-d was prepared. It was synthesized via aldol condensation of 4'-fluoro-2'-hydroxyacetophenone with appropriately substituted aldehydes followed by cyclization with hydrazine
M J Moonen et al.
Journal of industrial microbiology & biotechnology, 26(1-2), 35-42 (2001-09-11)
The biological Baeyer-Villiger oxidation of acetophenones was studied by 19F nuclear magnetic resonance (NMR). The 19F NMR method was used to characterise the time-dependent conversion of various fluorinated acetophenones in either whole cells of Pseudomonas fluorescens ACB or in incubations
Journal of Heterocyclic Chemistry, 30, 445-445 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门