所有图片(1)
About This Item
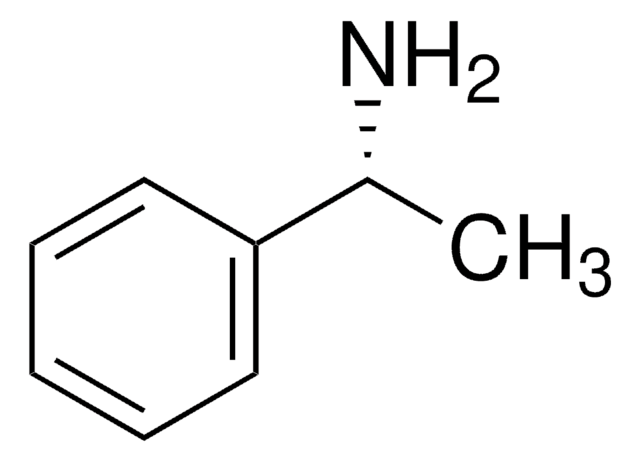
线性分子式:
[C6H5CH(CH3)]2NH
CAS号:
分子量:
225.33
Beilstein:
3590931
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
光學活性
[α]20/D +199.0°, neat
光學純度
ee: ≥99% (GLC)
折射率
n20/D 1.5523 (lit.)
bp
86 °C/0.05 mmHg (lit.)
密度
0.985 g/mL at 25 °C (lit.)
官能基
amine
phenyl
SMILES 字串
C[C@@H](N[C@H](C)c1ccccc1)c2ccccc2
InChI
1S/C16H19N/c1-13(15-9-5-3-6-10-15)17-14(2)16-11-7-4-8-12-16/h3-14,17H,1-2H3/t13-,14-/m1/s1
InChI 密鑰
NXLACVVNHYIYJN-ZIAGYGMSSA-N
應用
(+)-双[(R)-1-苯基乙基]胺可用于:
- β-氨基酸的合成。
- 制备具有手性C(19)-C(26)和C(27)-C(32)部分的scytophycin C。
- 在前手性酮的去质子化中诱导对映选择性。
- 作为通过曼尼希缩合反应合成手性酚盐配体的原料。
- 合成手性亚磷酰胺配体。
- 制备手性环状异亚胺盐,其可进一步用于通过Diels-Alder反应合成手性内酯。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>235.4 °F - closed cup
閃點(°C)
> 113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Javier Francos et al.
Dalton transactions (Cambridge, England : 2003), 43(3), 1408-1412 (2013-11-10)
A study has been conducted to determine whether lithium magnesiates are feasible candidates for the enantioselective deprotonation of 4-alkylcyclohexanones. The commercially available chiral amine (+)-bis[(R)-1-phenylethyl]amine (2-H) was utilised to induce enantioselection. When transformed to its lithium salt and combined with
Robert K Boeckman et al.
Organic letters, 12(20), 4524-4527 (2010-09-17)
Diels-Alder reactions of cyclic isoimidium salts are described. The corresponding cycloadducts are obtained with high regio- and stereoselectivity. The use of homochiral cyclic isoimidium salts delivers cycloadducts with excellent diastereoselectivity (>99:1) that can be efficiently converted to enantiomerically pure lactones.
Davies, S.G. Ichihara, O.
Tetrahedron Asymmetry, 2, 183-183 (1991)
Synthesis and Characterization of New Chiral Monoanionic [ON] Ancillary Phenolate Ligands
Binda P, et al.
International Journal of Organic Chemistry, 4(03), 182-182 (2014)
Kevin W Hunt et al.
Organic letters, 4(2), 245-248 (2002-02-14)
[reaction: see text] An efficient strategy for transforming meso-oxabicyclo[3.2.1]octenone 1 into optically active intermediates for macrolide synthesis has been developed. The direct bridgehead opening of optically active oxabicyclo[3.2.1]octene derivative 2 with hydride or a silyl ketene acetal utilizing the highly
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门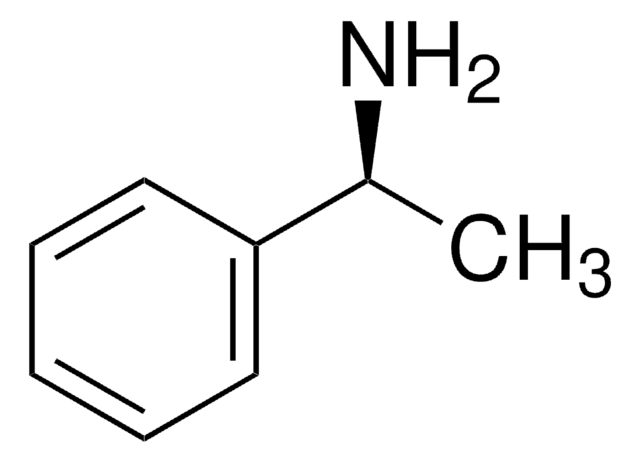
![(-)-双[(S)-1-苯基乙基]胺 99%](/deepweb/assets/sigmaaldrich/product/structures/336/455/d6f04f0e-9bcc-4d67-a94d-d153e39209e1/640/d6f04f0e-9bcc-4d67-a94d-d153e39209e1.png)