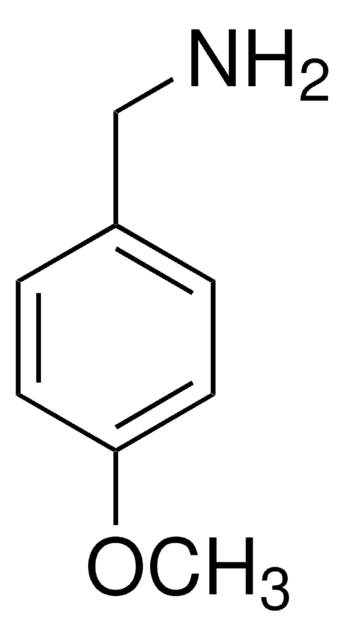
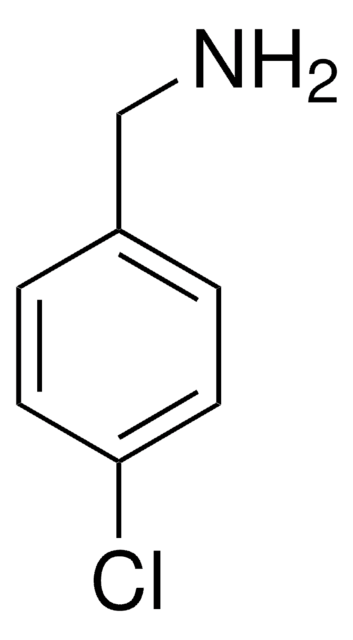
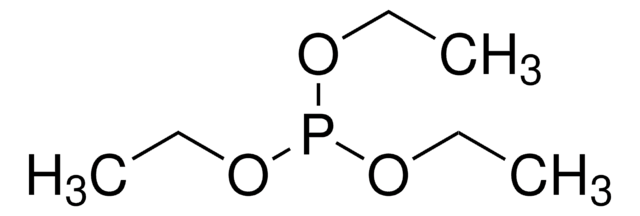
推荐产品
品質等級
化驗
98%
折射率
n20/D 1.549 (lit.)
bp
140 °C/1 mmHg (lit.)
密度
1.113 g/mL at 25 °C (lit.)
官能基
amine
SMILES 字串
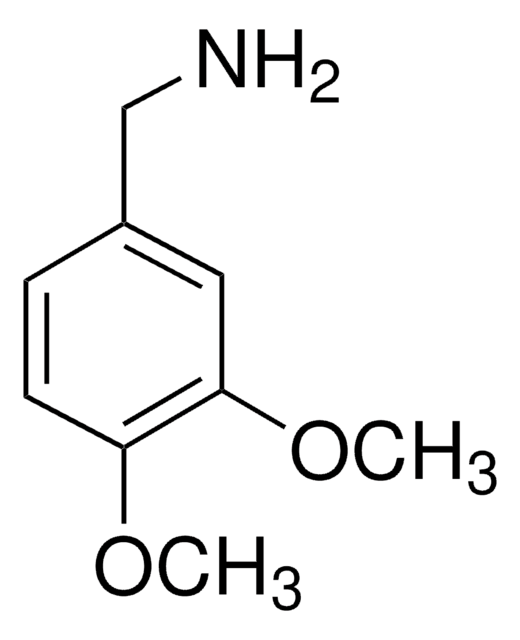
COc1ccc(CN)c(OC)c1
InChI
1S/C9H13NO2/c1-11-8-4-3-7(6-10)9(5-8)12-2/h3-5H,6,10H2,1-2H3
InChI 密鑰
QOWBXWFYRXSBAS-UHFFFAOYSA-N
一般說明
2,4-二甲氧基苄胺可以通过还原(NaBH4,BF3.OEt2,,THF)2,4-二甲氧基苄腈来制备。
應用
2,4-二甲氧基苄胺是一种胺亲核试剂,用于研究5-溴-2-茚-1-酮的 1,4- 反应性。它可用于以下研究:
- 作为一系列 2,4,5-三取代恶唑的简明合成中的氨当量,通过串联 Ugi/Robinson-Gabriel 反应序列。
- 全合成(-)-muraymycin(MRY)D2 及其差向异构体,抗菌核苷天然产物。
- 使用 Ugi 反应,两步法合成尿嘧啶多氧霉素 C(UPOC)甲酯的酰胺衍生物。
- N-羟基硫脲的合成。
- 合成抗 HIV-1 试剂。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
M Sato et al.
Journal of medicinal chemistry, 19(2), 336-337 (1976-02-01)
The synthesis of the title compound (1) was accomplished by the conversion of 2,4-dimethoxybenzylamine (2) into an isothiocyanate (3) using thiocarbonyl diimidazole. Treatment of 3 with hydroxylamine and removal of the DMB group with trifluoroacetic acid gave 1. N-Hydroxythiourea (1)
Arthur Y Shaw et al.
Tetrahedron letters, 53(15), 1998-2000 (2013-04-06)
This Letter discloses a novel concise synthesis of a series of 2,4,5-trisubstituted oxazoles via a tandem Ugi/Robinson-Gabriel sequence. Herein, 2,4-dimethoxybenzylamine
Tetsuya Tanino et al.
The Journal of organic chemistry, 75(5), 1366-1377 (2010-02-11)
Full details of the first total synthesis of (-)-muraymycin (MRY) D2 and its epimer, the antibacterial nucleoside natural product, are described. Key strategic elements of the approach include the preparation of the urea dipeptide moiety found in the muraymycins containing
Tetrahedron Letters, 47, 8459-8459 (2006)
Keith J Stanger et al.
Journal of combinatorial chemistry, 8(3), 435-439 (2006-05-09)
We describe parallel/combinatorial, solid-phase, supported synthesis of diverse hydroxamates using a common intermediate, an N-derivatized, O-linked hydroxylamine. The method allows the concurrent synthesis of both N-alkyl and N-H hydroxamates and is compatible with a wide range of chemical transformations. The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持