所有图片(2)
About This Item
线性分子式:
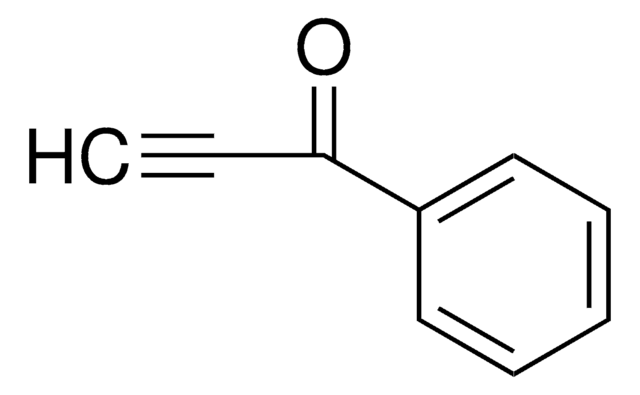
CH3C6H4SO2C≡CH
CAS号:
分子量:
180.22
Beilstein:
2556169
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
73-74 °C (lit.)
溶解度
organic solvents: soluble(lit.)
官能基
sulfone
SMILES 字串
Cc1ccc(cc1)S(=O)(=O)C#C
InChI
1S/C9H8O2S/c1-3-12(10,11)9-6-4-8(2)5-7-9/h1,4-7H,2H3
InChI 密鑰
FTHKWIMQNXVEHW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Ethynyl p-tolyl sulfone (Tosylacetylene) may be used in the synthesis of 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) protected derivatives. It may be used as starting reagent for the synthesis of optically active indan-2-ols.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Takeshi Hanazawa et al.
The Journal of organic chemistry, 68(12), 4980-4983 (2003-06-07)
An efficient and practical synthesis of optically active indan-2-ols 1 has been developed starting from readily accessible optically active 4-siloxy-1,6-alkadiynes 2 and ethynyl p-tolyl sulfone, where the metalative Reppe reaction mediated by an economical divalent titanium reagent, Ti(O-i-Pr)(4)/2 i-PrMgCl, is
A Simplified Method for the Preparation of Ethynyl P-Tolyl Sulfone and Ethynyl Phenyl Sulfone.
Chen Z and Trudell ML.
Synthetic Communications, 24(21), 3149-3155 (1994)
David Tejedor et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(66), 15046-15049 (2019-09-26)
A universal, practical and scalable organocatalytic hydrocyanation manifold to provide β-substituted acrylonitriles bearing an electron-withdrawing functionality has been implemented. The catalytic manifold operates under the reactivity generation principle "a good nucleophile generates a strong base", and it uses 1,4-diazabicyclo[2.2.2]octane (DABCO)
Elena Petit et al.
The Journal of organic chemistry, 79(18), 8826-8834 (2014-08-28)
The use of the 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) group for the protection of the NH group of a series of imides, azinones (including AZT), inosines, and cyclic sulfonamides has been examined. The Tsv-protected derivatives are obtained in excellent yields by conjugate
Hao Chen et al.
Bioorganic & medicinal chemistry letters, 17(7), 1979-1983 (2007-02-16)
A library of potential antifungal triazole-modified beta-methoxyacrylate analogues was designed and synthesized via a Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction or 'click chemistry'. Subsequent biological screening revealed that some compounds displayed low to moderate antifungal activity toward pathogenic fungi and low
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门