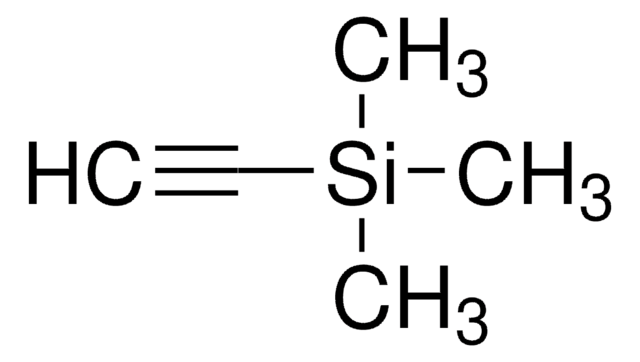所有图片(1)
About This Item
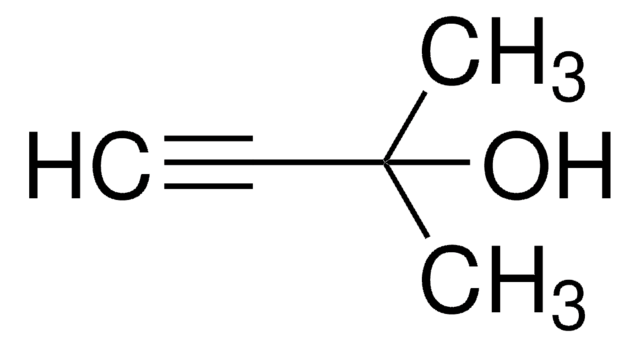
线性分子式:
(CH3)3CSi(CH3)2C≡CH
CAS号:
分子量:
140.30
Beilstein:
1921466
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
折射率
n20/D 1.451 (lit.)
bp
116-117 °C (lit.)
密度
0.751 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)(C)[Si](C)(C)C#C
InChI
1S/C8H16Si/c1-7-9(5,6)8(2,3)4/h1H,2-6H3
InChI 密鑰
RTYNRTUKJVYEIE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(tert-Butyldimethylsilyl)acetylene is a bulky trialkylsilyl-protected alkyne. It participates in Cadiot-Chodkiewicz cross-coupling reaction with various bromoalkynes to afford synthetically useful unsymmetrical diynes.
(tert-Butyldimethylsilyl)acetylene readily undergoes cross-dimerization reaction with various internal phenyl acetylenes in the presence of rhodium dimers and bidentate phosphine ligands to afford enynes.
應用
(tert-Butyldimethylsilyl)acetylene may be used in the synthesis of β-alkynylketone and β-alkynyl aldehydes.
其他客户在看
Asymmetric synthesis of beta-alkynyl aldehydes by rhodium-catalyzed conjugate alkynylation.
Takahiro Nishimura et al.
Angewandte Chemie (International ed. in English), 48(43), 8057-8059 (2009-09-22)
Trost BM and Li CJ.
Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (2014)
Joseph P Marino et al.
The Journal of organic chemistry, 67(19), 6841-6844 (2002-09-14)
Bulky trialkylsilyl-protected alkynes such as triethylsilyl (TES), tert-butyldimethylsilyl (TBS), and triisopropylsilyl (TIPS) acetylenes underwent the Cadiot-Chodkiewicz cross-coupling reaction with different bromoalkynes to form a variety of synthetically useful unsymmetrical diynes in good yields. The diyne alcohol 10 was transformed regio-
Steric tuning of silylacetylenes and chiral phosphine ligands for rhodium-catalyzed asymmetric conjugate alkynylation of enones.
Takahiro Nishimura et al.
Journal of the American Chemical Society, 130(5), 1576-1577 (2008-01-17)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门