所有图片(1)
About This Item
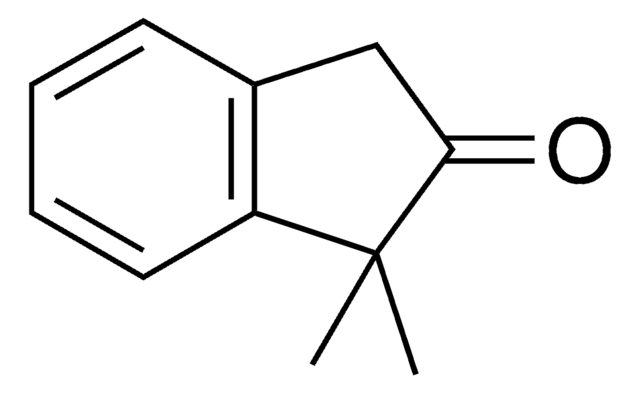
经验公式(希尔记法):
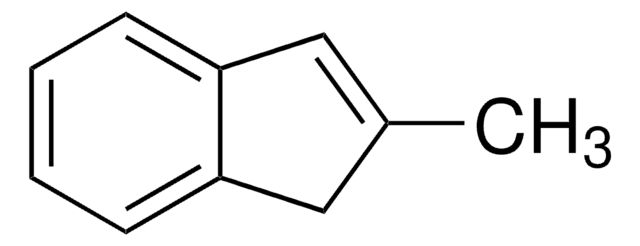
C10H10O
CAS号:
分子量:
146.19
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.555 (lit.)
bp
93-95 °C/4 mmHg (lit.)
密度
1.064 g/mL at 25 °C (lit.)
官能基
ketone
SMILES 字串
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
InChI 密鑰
BEKNOGMQVKBMQN-UHFFFAOYSA-N
一般說明
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
應用
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction.
Luhowy R and Keehn PM.
Journal of the American Chemical Society, 99(11), 3797-3805 (1977)
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen.
Riahi A, et al.
New. J. Chem., 37(8), 2245-2249 (2013)
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
Selective and easy preparation of enol carbonates of α-disubstituted aryl ketones from their lithium enolates.
Aboulhoda SJ, et al.
Tetrahedron Letters, 36(27), 4795-4796 (1995)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门