推荐产品
化驗
99%
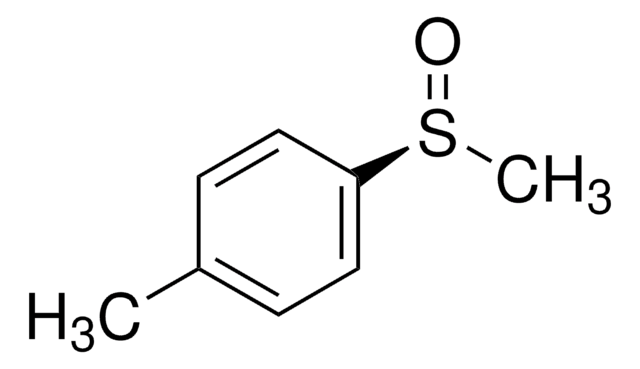
光學活性
[α]20/D +145°, c = 2 in acetone
光學純度
ee: 99% (HPLC)
mp
73-75 °C (lit.)
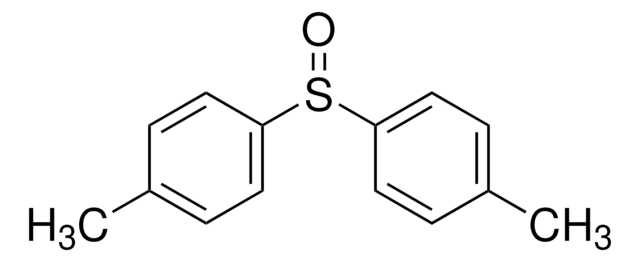
官能基
sulfoxide
SMILES 字串
Cc1ccc(cc1)S(C)=O
InChI
1S/C8H10OS/c1-7-3-5-8(6-4-7)10(2)9/h3-6H,1-2H3/t10-/m1/s1
InChI 密鑰
FEVALTJSQBFLEU-SNVBAGLBSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(R)-(+)-Methyl p-tolyl sulfoxide may be used to prepare (R)-(+)-methyl 3,5-dimethoxy-6-[8-oxo-9-(p-tolylsulfinyl) nonyl] benzoate, an intermediate for (R)-lasiodiplodin synthesis. Its anions undergo addition reaction with nitrones to form optically active a-substituted N-hydroxylamines. (R)-(+)-Methyl p-tolyl sulfoxide also reacts with O-mesitylsulfonylhydroxylamine (MSH) to form (-)-(R)-S-methyl-S-p-tolylsulfoximine.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Chemistry of sulfoxides and related compounds. XLIX. Synthesis of optically active sulfoximines from optically active sulfoxides.
Johnson CR, et al.
The Journal of Organic Chemistry, 39(16), 2458-2459 (1974)
Asymmetric synthesis of orsellinic acid type macrolides: The example of lasiodiplodin.
Solladie G, et al.
Tetrahedron Asymmetry, 1(3), 187-198 (1990)
The reaction of nitrones with (R)-(+)-methyl p-tolyl sulfoxide anion; asymmetric synthesis of optically active secondary amines.
Murahashi S-I, et al.
Tetrahedron Letters, 34(16), 2645-2648 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门