所有图片(3)
About This Item
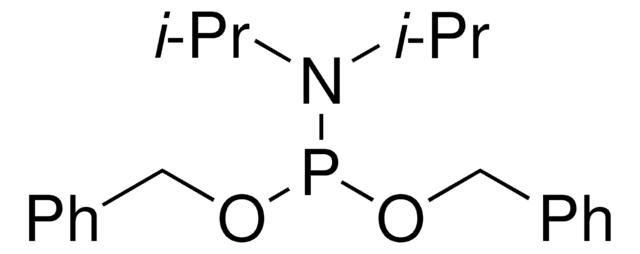
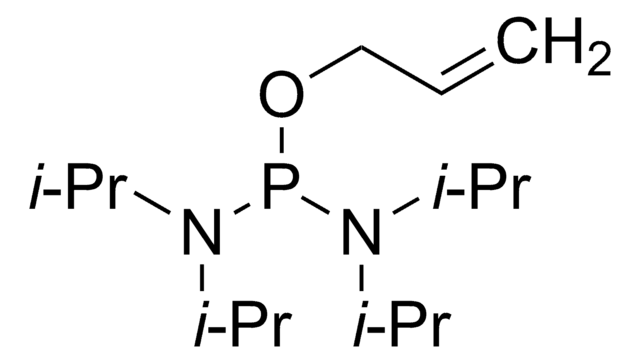
线性分子式:
{[(CH3)2CH]2N}2POCH2CH2CN
CAS号:
分子量:
301.41
Beilstein:
3590103
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.470 (lit.)
bp
100 °C/0.5 mmHg (lit.)
密度
0.949 g/mL at 25 °C (lit.)
官能基
amine
nitrile
儲存溫度
−20°C
SMILES 字串
CC(C)N(C(C)C)P(OCCC#N)N(C(C)C)C(C)C
InChI
1S/C15H32N3OP/c1-12(2)17(13(3)4)20(19-11-9-10-16)18(14(5)6)15(7)8/h12-15H,9,11H2,1-8H3
InChI 密鑰
RKVHNYJPIXOHRW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2-氰基乙基N,N,N′,N′-四异丙基亚磷酰二胺被用于:
- 制备合成12-聚体寡脱氧核苷酸所需的亚磷酰胺试剂
- 作为合成1,2-二酰基-sn-甘油磷脂酰丝氨酸的磷酸化试剂
- 脱氧核糖核苷亚磷酰胺的原位 制备
- 制备2′-脱氧-2′-氟-3′-O-(β-氰基乙基-N,N-二异丙基磷酰基)-5′-O-(4-甲氧基三苯甲基)-4′-硫代-β-D-阿拉伯尿苷和1-(3-O-(β-氰基乙基-N,N-二异丙基磷酰基)-2-脱氧-2-氟-5-O-(4,4′-二甲氧基三苯甲基)-4-硫代-β-D-阿拉伯糖)-胸腺嘧啶
- 作为亚磷酸化核苷酸的合成试剂
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Flam. Liq. 3 - Skin Sens. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
119.3 °F - closed cup
閃點(°C)
48.5 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Vyacheslav V Filichev et al.
Bioorganic & medicinal chemistry, 12(11), 2843-2851 (2004-05-15)
Hexofuranosyl nucleosides are considered as conformationally restricted acyclic nucleosides using a furanose ring to link the diol backbone to the nucleobase. The phosphoramidite of 1-(2,3-dideoxy-beta-D-erythro-hexofuranosyl)thymine was synthesized from thymidine with formation of a new stereocentre at C-5' and the nucleoside
Lubomir V Nechev et al.
Chemical research in toxicology, 15(5), 607-613 (2002-05-23)
3-(2-Deoxy-beta-D-erythro-pentofuranosyl)-6-hydroxy-5,6,7,8-tetrahydropyrimido[1,2-a]purin-10(3H)-one is formed in low yield by the reaction of acrolein with 2'-deoxyguanosine. The nucleoside and an oligodeoxynucleotide containing it have been synthesized. For preparation of the nucleoside 2'-deoxyguanosine was alkylated at the N1 position using 1-bromo-3-butene to give 1-(3-butenyl)-2'-deoxyguanosine.
Arvind Misra et al.
Bioconjugate chemistry, 15(3), 638-646 (2004-05-20)
Synthesis of modified oligonucleotides in which the specific cytidine nucleoside analogues linked at 2'-OH position via a carbamate bond with an amino ethyl derivative of dansyl fluorophore is reported. For the multiple labeling of oligonucleotides, a strategy involving prelabeling at
Improved synthesis of (Pri2 N)2POCH2CH2CN.
J Nielsen et al.
Nucleic acids research, 15(8), 3626-3626 (1987-04-24)
Jonathan K Watts et al.
Nucleic acids research, 35(5), 1441-1451 (2007-02-08)
The synthesis of oligonucleotides containing 2'-deoxy-2'-fluoro-4'-thioarabinonucleotides is described. 2'-Deoxy-2'-fluoro-5-methyl-4'-thioarabinouridine (4'S-FMAU) was incorporated into 18-mer antisense oligonucleotides (AONs). 4'S-FMAU adopts a predominantly northern sugar conformation. Oligonucleotides containing 4'S-FMAU, unlike those containing FMAU, were unable to elicit E. coli or human RNase
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门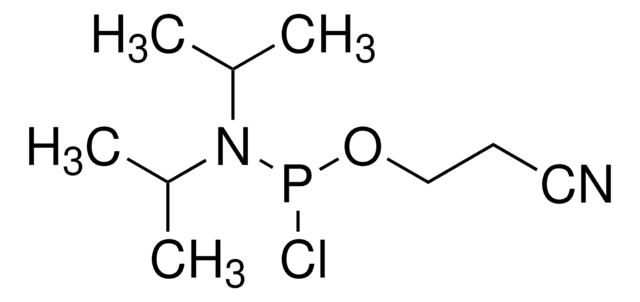



![Bis[2-(trimethylsilyl)ethyl] N,N-diisopropylphosphoramidite 96%](/deepweb/assets/sigmaaldrich/product/structures/666/768/dbdabf23-1642-4431-8e0d-32f726897297/640/dbdabf23-1642-4431-8e0d-32f726897297.png)
