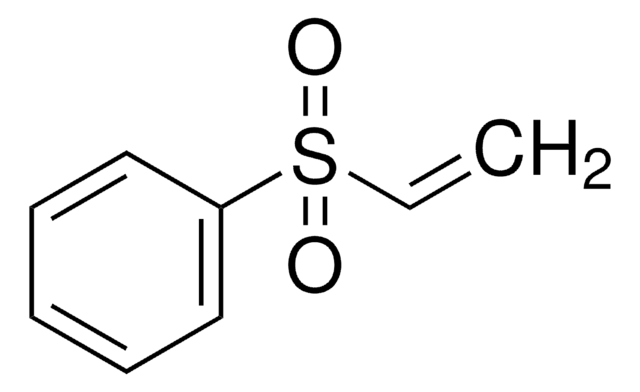
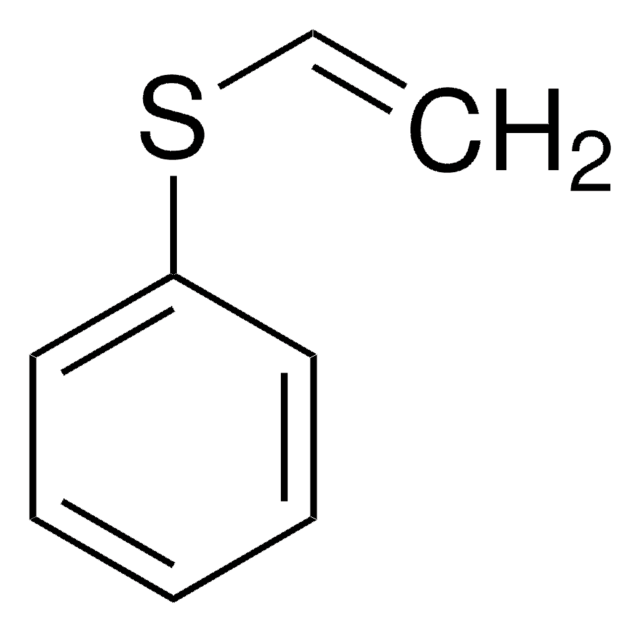
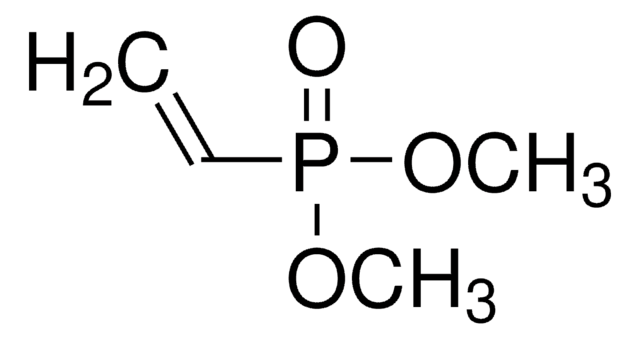
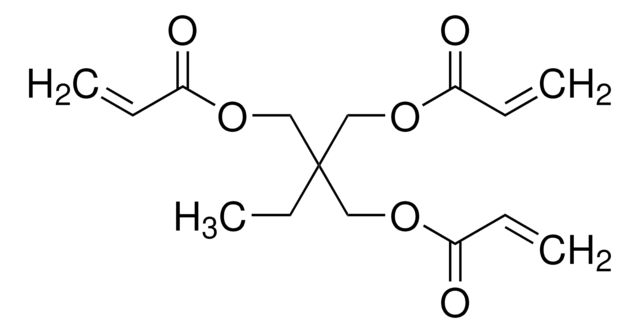
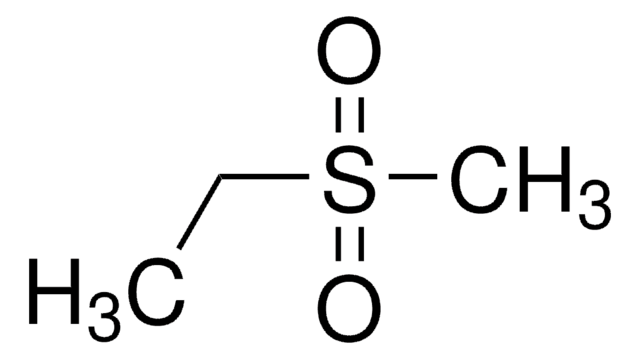
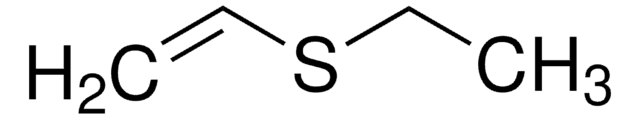推荐产品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.463 (lit.)
bp
118-119 °C/22 mmHg (lit.)
密度
1.151 g/mL at 25 °C (lit.)
SMILES 字串
CCS(=O)(=O)C=C
InChI
1S/C4H8O2S/c1-3-7(5,6)4-2/h3H,1,4H2,2H3
InChI 密鑰
BJEWLOAZFAGNPE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Ethly vinyl sulfone alkylates ε-amino groups of lysine side chains and imidazole groups of histidine residues in proteins. Chemical modification of bovine serum albumin by ethyl vinyl sulfone has been studied by X-ray photoelectron spectroscopy.
相關產品
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
228.2 °F - closed cup
閃點(°C)
109 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
M Friedman et al.
International journal of peptide and protein research, 7(6), 481-486 (1975-01-01)
Ethly vinyl sulfone (EVS) alkylates xi-amino groups of lysine side chains and imidazole groups of histidine residues in proteins. Amino acid analysis of hydrolyzates of EVS-treated polylysine shows that lysine forms two derivatives, presumably xi-N-(ethylsulfonylethyl)lysine and xi, xi, N,N-bis(ethylsulfonylethyl)lysine that
X-ray photoelectron spectroscopy of BSA and ethyl vinyl sulfone modified BSA.
M M Millard et al.
Biochemical and biophysical research communications, 70(2), 445-451 (1976-05-17)
M S Masri et al.
Journal of protein chemistry, 7(1), 49-54 (1988-02-01)
Disulfide bonds of bovine serum albumin and wool were reduced by n-tributylphosphine to sulfhydryl groups that were then modified by methyl or ethyl vinyl sulfone in a nucleophilic addition reaction to S-(beta-ethylsulfonylmethyl)-L-cysteine and S(beta-ethylsulfonylethyl)-L-cysteine, respectively. The reductive alkylation was carried
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门