所有图片(1)
About This Item
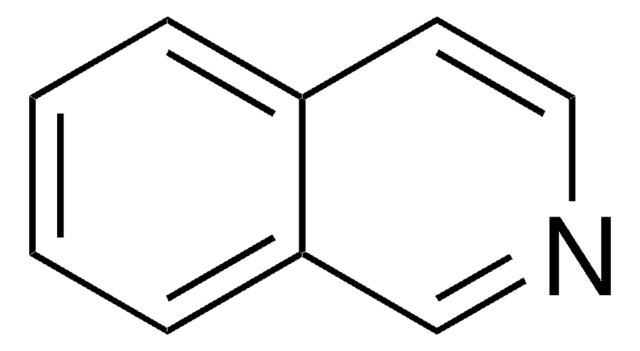
经验公式(希尔记法):
C13H7NO2
CAS号:
分子量:
209.20
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
mp
178-180 °C (lit.)
官能基
ketone
SMILES 字串
O=C1c2ccccc2C(=O)c3cnccc13
InChI
1S/C13H7NO2/c15-12-8-3-1-2-4-9(8)13(16)11-7-14-6-5-10(11)12/h1-7H
InChI 密鑰
ZLLVUAAESHIVAZ-UHFFFAOYSA-N
一般說明
Benz[g]isoquinoline-5,10-dione has been isolated as an active component from the ethanolic extract of the aerial parts of Mitracarpus scaber. It exhibits significant in vitro inhibitory activity against the AIDS-related pathogens. The in vitro antibacterial and anti-fungal activity of benz[g]isoquinoline-5,10-dione has been investigated using the agar well-diffusion assay.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Ekaterina Shinkevich et al.
Organic & biomolecular chemistry, 9(2), 538-548 (2010-10-27)
1,2-Disubstituted 1,2,3,4-tetrahydrobenz[g]isoquinoline-5,10-diones are prepared for the first time through an activated Pictet-Spengler reaction of the corresponding imines of 2-(1,4-dimethoxynaphth-2-yl)ethylamine in the presence of an acyl chloride and AlCl(3) followed by an oxidation with silver(II) oxide in nitric acid. Depending on
Andrew Jonathan Nok
Cell biochemistry and function, 20(3), 205-212 (2002-07-19)
An ethanolic extract of Mitracarpus scaber was found to possess in vitro and in vivo trypanocidal activity against Trypanosoma congolense. At a dosage of 50 mg kg(-1) day(-1) in normal saline for 5 days, the extract cured Balbc mice infected
A L Okunade et al.
Planta medica, 65(5), 447-448 (1999-07-27)
An ethanolic extract of the aerial parts of Mitracarpus scaber demonstrated good antimicrobial activity. Bioassay directed fractionation of this extract led to the isolation of benz[g]isoquinoline-5,10-dione (1) as an active component. Compound 1 showed significant in vitro inhibitory activity against
B T Walton et al.
Science (New York, N.Y.), 222(4622), 422-423 (1983-10-28)
Morphological abnormalities including extra compound eyes, extra heads, and distally duplicated legs were generated in cricket embryos by treating eggs with single doses of either benz[g]isoquinoline-5,10-dione or benzo[h]quinoline-5,6-dione. Slight structural modifications of the molecules resulted in a loss of teratogenic
A M Clark et al.
Pharmaceutical research, 1(6), 269-271 (1984-11-01)
The in vitro antibacterial and anti-fungal activity of benz[g]isoquinoline-5,10-dione (1), benzo[g]quinoline-5, 10-dione (2), benzo[g]quinoline-5,6-dione (3), and anthraquinone (4) was determined using the agar well-diffusion assay. The minimum inhibitory concentrations (MIC's) of each of the active compounds (1-3) was determined using
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门


![1H-苯并[g]吲哚 97%](/deepweb/assets/sigmaaldrich/product/structures/568/798/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd/640/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd.png)