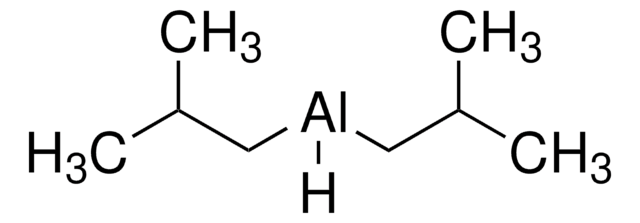
推荐产品
形狀
liquid
反應適用性
reagent type: reductant
濃度
1.0 M in heptane
密度
0.731 g/mL at 25 °C
SMILES 字串
CC(C)C[AlH]CC(C)C
InChI
1S/2C4H9.Al.H/c2*1-4(2)3;;/h2*4H,1H2,2-3H3;;
InChI 密鑰
AZWXAPCAJCYGIA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用于Pd催化的仲烷基溴的还原脱溴过程。过苄基化呋喃糖苷的O-脱苄基和开环。方便从 ZrCp2Cl2 和DIBAL-H原位生成 HZrCp2Cl。
推薦產品
建议使用弯通式隔垫入口接头 Z118206 或直通式隔垫入口接头 Z118141(6mm 内径入口)或直通式隔垫入口接头 Z118192(13mm 内径入口)。
隔膜進氣口介面卡
訊號詞
Danger
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
標靶器官
Central nervous system
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Damien Webb et al.
Organic letters, 14(2), 568-571 (2011-12-31)
A continuous flow system for the multiparameter (flow rate, temperature, residence time, stoichiometry) optimization of the DIBALH reduction of esters to aldehydes is described. Incorporating an in-line quench (MeOH), these transformations are generally complete in fewer than 60 s. Mixing
Hidetsura Cho et al.
The Journal of organic chemistry, 75(3), 627-636 (2009-12-31)
A systematic investigation of the reductive ring-expansion reaction of cyclic ketoximes fused to aromatic rings with diisobutylaluminum hydride (DIBALH) is described. This reaction regioselectively afforded a variety of five- to eight-membered bicyclic heterocycles or tricyclic heterocycles containing nitrogen neighboring an
D J Kopecky et al.
The Journal of organic chemistry, 65(1), 191-198 (2000-05-18)
An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding alpha-acetoxy ethers in good to excellent yields. It was
J Marco-Contelles et al.
Carbohydrate research, 335(1), 63-70 (2001-09-13)
The reaction of DIBALH with bis(heteroannulated)-pyranosides containing the perhydrofuro[2,3-b]pyran moiety is described. The hydride attack at the anomeric carbon (C-9a) resulted in the exclusive tetrahydrofuran ring opening. The selectivity of this reaction has been evaluated as other benzylidene acetals built
Y Kitade et al.
Nucleic acids symposium series, (27)(27), 107-108 (1992-01-01)
Reaction of purine nucleosides, such as 2',3'-isopropylideneinosine (1a) and 2',3'-isopropylideneadenosine (1c), with diisobutylaluminum hydride (DIBAL) in dry tetrahydrofurane resulted in the formation of the corresponding 9-(2',3'-isopropylideneribity)purines (2) in good yields. Oxidation of the ribityl derivatives (2) with NalO4 and subsequent
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门