推荐产品
化驗
≥97%
形狀
solid
mp
59-61 °C (lit.)
SMILES 字串
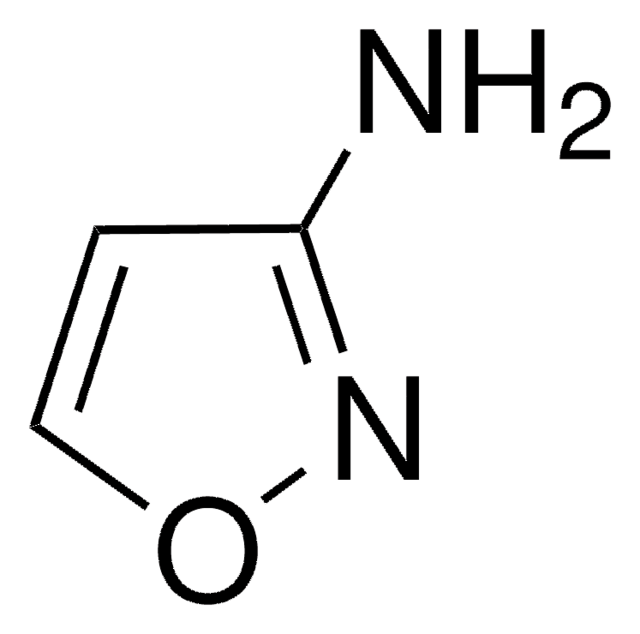
Cc1cc(N)no1
InChI
1S/C4H6N2O/c1-3-2-4(5)6-7-3/h2H,1H3,(H2,5,6)
InChI 密鑰
FKPXGNGUVSHWQQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-氨基-5-甲基异恶唑是 嗜冷假单胞菌 菌株 HA-4 在磺胺甲恶唑生物降解过程中形成的主要中间体。它是磺胺甲恶唑 (SMX) 光催化降解过程中形成的中间体 。
應用
3-氨基-5-甲基异恶唑用于合成:
- 萘并[1,2-e][1,3]恶嗪
- 1-芳基-4-甲基-3,6- 双 -(5-甲基异恶唑-3-基)-2-硫代-2,3,6,10 b -四氢-1 H -嘧啶并[5,4- c ] ] 喹啉-5-酮系列,具有潜在的杀蚊杀幼活性
- 磺胺嘧啶和硫胺甲恶唑的羟胺
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Lu Wang et al.
Water research, 88, 322-328 (2015-10-30)
Sulphamethoxazole (SMX) is extensively used in humans and livestock, but its appearance in natural water raises environmental concerns. This study demonstrated that SMX and its degradation product, 3-amino-5-methylisoxazole (3A5MI), could be effectively degraded in microbial fuel cell (MFC) reactors. Approximately
Shiyuan Ding et al.
Journal of hazardous materials, 262, 812-818 (2013-10-22)
Photocatalytic degradation of sulfamethoxazole (SMX) was investigated using Bi2O3/Bi2O2CO3/Sr6Bi2O9 (BSO) photocatalyst under visible light (>420 nm) irradiation. The photochemical degradation of SMX followed pseudo-first-order kinetics. The reaction kinetics was determined as a function of initial SMX concentrations (5-20 mg L(-1))
Benchao Jiang et al.
Applied microbiology and biotechnology, 98(10), 4671-4681 (2014-02-14)
Sulfamethoxazole is a common antibiotic that is frequently detected in wastewater and surface water. This study investigated the biodegradation and metabolic pathway of sulfamethoxazole by Pseudomonas psychrophila HA-4, a cold-adapted bacterium. Strain HA-4, which uses sulfamethoxazole as its sole source
Mehdi Shafiee et al.
Molecular diversity, 16(4), 727-735 (2012-10-24)
An expeditious, straightforward and efficient synthesis of diversely naphtho[1,2-e][1,3]oxazines via one-pot condensation reaction of β- naphthol, 3-amino-5-methylisoxazole and arylaldehydes catalyzed by bismuth(III) trifluoromethanesulfonate is described. The reaction preferentially afforded 1,3-trans oxazines.
Bianca M Souza et al.
Environmental science and pollution research international, 24(7), 6195-6204 (2015-11-12)
The present study aims to assess the removal of 3-amino-5-methylisoxazole (AMI), a recalcitrant by-product resulting from the biological breakdown of some pharmaceuticals, applying a solar photo-Fenton process assisted by ferrioxalate complexes (SPFF) (Fe
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门