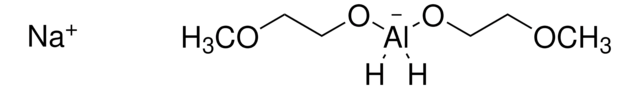
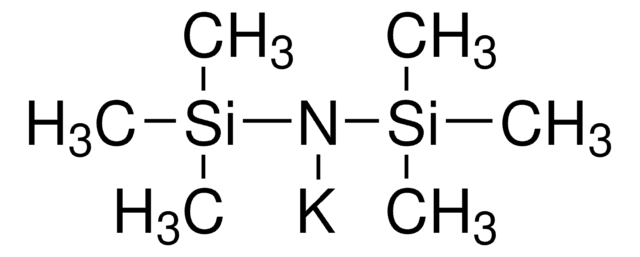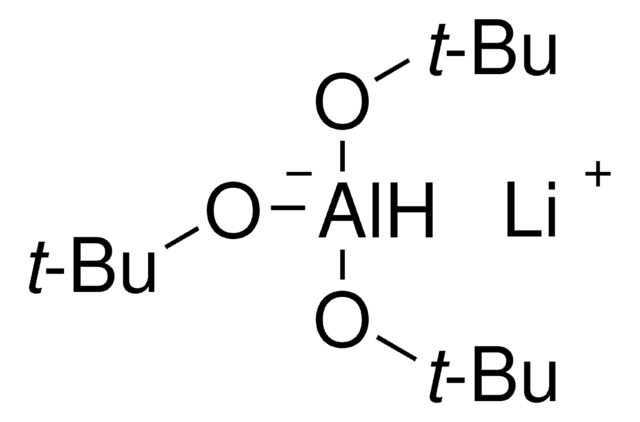推荐产品
形狀
liquid
品質等級
反應適用性
reagent type: reductant
濃度
2.0 M in THF
密度
0.896 g/mL at 25 °C
SMILES 字串
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI 密鑰
UUKMSDRCXNLYOO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
反应物用于:
- 镓、铟、铼和锌三(巯基咪唑基)氢硼酸配合物的制备
- 机械化学复分解反应
- 非催化水解制氢
- 大型金单层保护簇的生长
- 阴离子取代反应
- 脱氢反应
硼氢化锂溶液 (2M in THF) 分别用于 5-亚苄基-2,4-噻唑烷二酮类和 5-亚苄基-4-氧代-2-噻唑烷二酮类的还原,并分别生成 5-苄基-2,4-噻唑烷二酮类和 5-苄基-4-氧代-2-噻唑烷二酮类。
包裝
推荐将25毫升安全/密封™瓶作为一次性使用的瓶子。重复穿刺可能会导致产品性能下降。
法律資訊
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 2
閃點(°F)
-0.4 °F - closed cup
閃點(°C)
-18 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Regiospecific reduction of 5-benzylidene-2, 4-thiazolidinediones and 4-oxo-2-thiazolidinethiones using lithium borohydride in pyridine and tetrahydrofuran.
Giles RG
Tetrahedron, 56(26), 4531-4537 (2000)
G Váradi et al.
International journal of peptide and protein research, 43(1), 29-30 (1994-01-01)
For solid-phase peptide synthesis, 2,4-dimethoxy-4'-hydroxbenzhydrol linker was prepared via lithium borohydride reduction of 2,4-dimethoxy-4'-hydroxybenozophenone. The potassium salt of the linker was coupled to chloromethylpolystyrene. This method proved to be better than use of the cesium salt. This new synthesis gave
Barbara Milani et al.
Dalton transactions (Cambridge, England : 2003), (34)(34), 4659-4663 (2008-11-26)
Transfer hydrogenation from 2-propanol to CO/4-methylstyrene and CO/styrene polyketones was catalyzed by [Ir(diene)(N-N)X] (N-N = nitrogen chelating ligand; X = halogen) in the presence of a basic cocatalyst. The reactions were performed using dioxane as cosolvent, in order to overcome
N P Arbatskiĭ et al.
Bioorganicheskaia khimiia, 26(1), 51-60 (2000-05-12)
By the example of fetuin and a blood-group-specific mucin from porcine stomach, we showed that, under conditions of reductive degradation of glycoproteins with LiBH4-LiOH in 70% aqueous tert-butyl alcohol, the reduction and cleavage of amide bonds occur much faster than
Prabhat Arya et al.
Journal of combinatorial chemistry, 6(1), 54-64 (2004-01-13)
A diversity-oriented solution and solid-phase synthesis of tetrahydroquinoline-based tricyclic derivatives has been achieved from enantiomerically pure, natural product-like bicyclic scaffold. The solution synthesis of enantiopure bicyclic scaffold was developed by asymmetric hetero Michael reaction. Our approach for the synthesis of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门