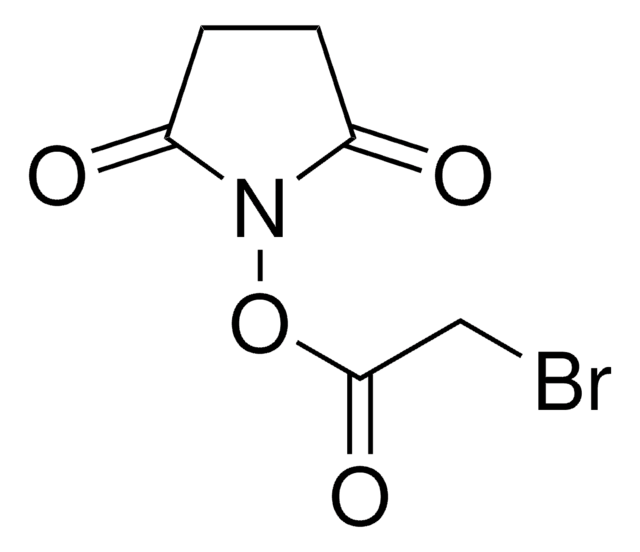
推荐产品
蒸汽壓力
<0.75 mmHg ( 20 °C)
品質等級
化驗
95%
形狀
solid
bp
120-123 °C/20 mmHg (lit.)
mp
48-60 °C (lit.)
溶解度
chloroform: soluble 100 mg/mL, clear, colorless to faintly yellow
官能基
anhydride
chloro
ester
SMILES 字串
ClCC(=O)OC(=O)CCl
InChI
1S/C4H4Cl2O3/c5-1-3(7)9-4(8)2-6/h1-2H2
InChI 密鑰
PNVPNXKRAUBJGW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
氯乙酸酐已用于合成:
- 3,3′-双(磺酸根)-4,4′-双(氯乙酰氨基)偶氮苯(BSBCA),一种水溶性、,硫醇反应性和光控交联剂
- D,L-7-氮杂色氨酸(D,L-7AW)
- 2-甲基-[3,4-二-O-乙酰基-6-O-(氯乙酰基)-1,2-二脱氧-α-D-连翘苷] - [2′,1′:4,5]-2-唑啉
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
289.4 °F - closed cup
閃點(°C)
143 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
K L Matta et al.
Carbohydrate research, 51(2), 215-222 (1976-11-01)
The use of the chloroacetyl group as a protecting group has been studied for a 2-methylglyco-[2',1':4,5]-2-oxazoline. The reaction of chloroacetyl chloride or chloroacetic anhydride with 2-acetamido-1,3,4-tri-O-acetyl-2-deoxy-beta-D-glucopyranose provided 2-acetamido-1,3,4-tri-O-acetyl-6-O-(chloroacetyl)-2-deoxy-beta-D-glucopyranose which, on treatment with anhydrous ferric chloride in dichloromethane, produced the desired
J D Brennan et al.
Analytical biochemistry, 252(2), 260-270 (1997-11-05)
The reaction of D,L-7-azatryptophan (D,L-7AW) with tryptophanyl-tRNA synthetase (TrpRS), adenosine triphosphate (ATP), and Mg2+ in the presence of inorganic pyrophosphatase results in the formation of a highly fluorescent l-7AW-adenylate complex. Detection of this complex is based on its enhanced fluorescence
Darcy C Burns et al.
Nature protocols, 2(2), 251-258 (2007-04-05)
This protocol describes a procedure for the synthesis of 3,3'-bis(sulfonato)-4,4'-bis(chloroacetamido)azobenzene (BSBCA), a water-soluble, thiol-reactive, photo-switchable cross-linker. In addition, a protocol is outlined for installing the cross-linker in an intramolecular fashion onto proteins bearing two surface-exposed Cys residues. BSBCA is designed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)