推荐产品
品質等級
化驗
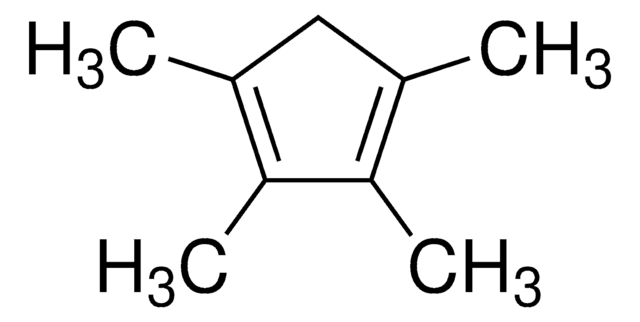
95%
折射率
n20/D 1.474 (lit.)
bp
58 °C/13 mmHg (lit.)
密度
0.87 g/mL at 25 °C (lit.)
SMILES 字串
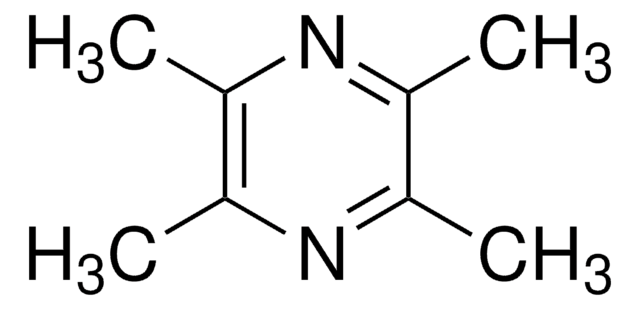
CC1C(C)=C(C)C(C)=C1C
InChI
1S/C10H16/c1-6-7(2)9(4)10(5)8(6)3/h6H,1-5H3
InChI 密鑰
WQIQNKQYEUMPBM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 五羰基铁中的金属有机溶剂蒸汽沉积中的生长调节剂化学品。
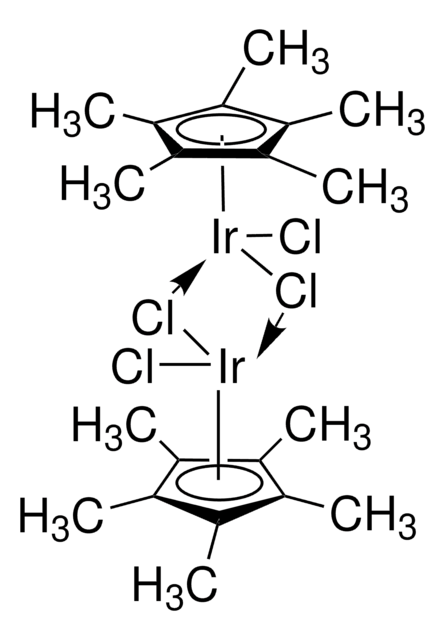
- 通过肟的中间体将醇催化转化为酰胺的“一锅法”铱中的配体。
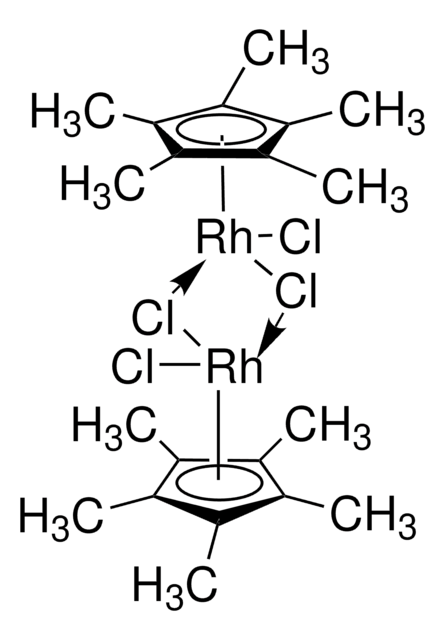
- 合成[Cp*Rh(bpy)H2O]2+(Cp* = 五甲基环戊二烯基,bpy = 2,2′-联吡啶),它是 NADH 再生过程中的电子介质。
其他客户在看
商品
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门