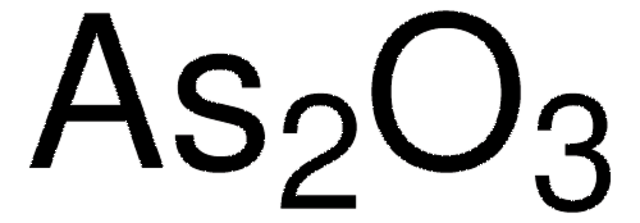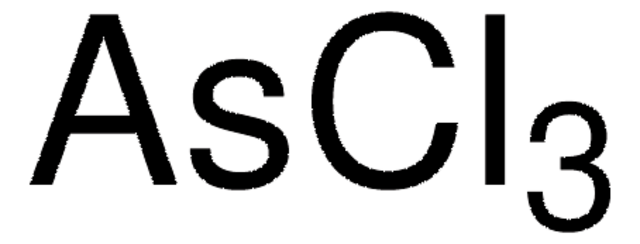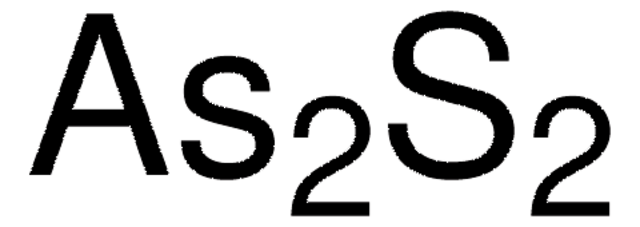推荐产品
品質等級
化驗
99.999% trace metals basis
電阻係數
33.3 μΩ-cm
bp
613 °C (lit.)
mp
817 °C (lit.)
密度
5.727 g/mL at 25 °C (lit.)
SMILES 字串
[As]
InChI
1S/As
InChI 密鑰
RQNWIZPPADIBDY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
砷片可用于制备α-雄黄(As4S4)。
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Repr. 1A - STOT RE 1 Oral
標靶器官
Respiratory organs,Cardio-vascular system,Gastro-intestinal system,Central nervous system,Nervous system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Valence and core-level binding energy shifts in realgar (As 4 S 4) and pararealgar (As 4 S 4) arsenic sulfides.
Bullen HA, et al.
Surface Science, 531(3), 319-328 (2003)
Claes Bergqvist et al.
Environmental pollution (Barking, Essex : 1987), 184, 540-546 (2013-11-05)
The toxicity of arsenic (As) in the environment is controlled by its concentration, availability and speciation. The aims of the study were to evaluate the accumulation and speciation of As in carrot, lettuce and spinach cultivated in soils with various
M Azizur Rahman et al.
Aquatic toxicology (Amsterdam, Netherlands), 146, 212-219 (2013-12-11)
Arsenic (As) is extremely toxic to living organisms at high concentration. In aquatic systems, As exists in different chemical forms. The two major inorganic As (iAs) species are As(V), which is thermodynamically stable in oxic waters, and As(III), which is
Lucia Cavalca et al.
Future microbiology, 8(6), 753-768 (2013-04-17)
Arsenic is present in many environments and is released by various natural processes and anthropogenic actions. Although arsenic is recognized to cause a wide range of adverse health effects in humans, diverse bacteria can metabolize it by detoxification and energy
Arsenic binding to proteins.
Shengwen Shen et al.
Chemical reviews, 113(10), 7769-7792 (2013-07-03)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门